Rad9 interacts with Aft1 to facilitate genome surveillance in fragile genomic sites under non-DNA damage-inducing conditions in S. cerevisiae
- PMID: 25300486
- PMCID: PMC4227768
- DOI: 10.1093/nar/gku915
Rad9 interacts with Aft1 to facilitate genome surveillance in fragile genomic sites under non-DNA damage-inducing conditions in S. cerevisiae
Abstract
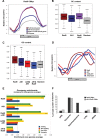
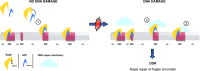
DNA damage response and repair proteins are centrally involved in genome maintenance pathways. Yet, little is known about their functional role under non-DNA damage-inducing conditions. Here we show that Rad9 checkpoint protein, known to mediate the damage signal from upstream to downstream essential kinases, interacts with Aft1 transcription factor in the budding yeast. Aft1 regulates iron homeostasis and is also involved in genome integrity having additional iron-independent functions. Using genome-wide expression and chromatin immunoprecipitation approaches, we found Rad9 to be recruited to 16% of the yeast genes, often related to cellular growth and metabolism, while affecting the transcription of ∼2% of the coding genome in the absence of exogenously induced DNA damage. Importantly, Rad9 is recruited to fragile genomic regions (transcriptionally active, GC rich, centromeres, meiotic recombination hotspots and retrotransposons) non-randomly and in an Aft1-dependent manner. Further analyses revealed substantial genome-wide parallels between Rad9 binding patterns to the genome and major activating histone marks, such as H3K36me, H3K79me and H3K4me. Thus, our findings suggest that Rad9 functions together with Aft1 on DNA damage-prone chromatin to facilitate genome surveillance, thereby ensuring rapid and effective response to possible DNA damage events.
© The Author(s) 2014. Published by Oxford University Press on behalf of Nucleic Acids Research.
Figures


Similar articles
-
Distinct associations of the Saccharomyces cerevisiae Rad9 protein link Mac1-regulated transcription to DNA repair.Curr Genet. 2020 Jun;66(3):531-548. doi: 10.1007/s00294-019-01047-w. Epub 2019 Nov 29. Curr Genet. 2020. PMID: 31784768
-
Functional genomics analysis of the Saccharomyces cerevisiae iron responsive transcription factor Aft1 reveals iron-independent functions.Genetics. 2010 Jul;185(3):1111-28. doi: 10.1534/genetics.110.117531. Epub 2010 May 3. Genetics. 2010. PMID: 20439772 Free PMC article.
-
A Rad53 independent function of Rad9 becomes crucial for genome maintenance in the absence of the Recq helicase Sgs1.PLoS One. 2013 Nov 20;8(11):e81015. doi: 10.1371/journal.pone.0081015. eCollection 2013. PLoS One. 2013. PMID: 24278365 Free PMC article.
-
Mechanisms of iron sensing and regulation in the yeast Saccharomyces cerevisiae.World J Microbiol Biotechnol. 2017 Apr;33(4):75. doi: 10.1007/s11274-017-2215-8. Epub 2017 Mar 17. World J Microbiol Biotechnol. 2017. PMID: 28315258 Review.
-
Role of the Saccharomyces cerevisiae Rad9 protein in sensing and responding to DNA damage.Biochem Soc Trans. 2003 Feb;31(Pt 1):242-6. doi: 10.1042/bst0310242. Biochem Soc Trans. 2003. PMID: 12546694 Review.
Cited by
-
Monitoring the prolonged Tnf stimulation in space and time with topological-functional networks.Comput Struct Biotechnol J. 2020 Jan 18;18:220-229. doi: 10.1016/j.csbj.2020.01.001. eCollection 2020. Comput Struct Biotechnol J. 2020. PMID: 32021663 Free PMC article.
-
Slx4 and Rtt107 control checkpoint signalling and DNA resection at double-strand breaks.Nucleic Acids Res. 2016 Jan 29;44(2):669-82. doi: 10.1093/nar/gkv1080. Epub 2015 Oct 20. Nucleic Acids Res. 2016. PMID: 26490958 Free PMC article.
-
Distinct chromosomal "niches" in the genome of Saccharomyces cerevisiae provide the background for genomic innovation and shape the fate of gene duplicates.NAR Genom Bioinform. 2022 Nov 14;4(4):lqac086. doi: 10.1093/nargab/lqac086. eCollection 2022 Dec. NAR Genom Bioinform. 2022. PMID: 36381424 Free PMC article.
-
Developmental conservation of microRNA gene localization at the nuclear periphery.PLoS One. 2019 Nov 4;14(11):e0223759. doi: 10.1371/journal.pone.0223759. eCollection 2019. PLoS One. 2019. PMID: 31682635 Free PMC article.
-
Mrc1 and Rad9 cooperate to regulate initiation and elongation of DNA replication in response to DNA damage.EMBO J. 2018 Nov 2;37(21):e99319. doi: 10.15252/embj.201899319. Epub 2018 Aug 29. EMBO J. 2018. PMID: 30158111 Free PMC article.
References
-
- Kerzendorfer C., O'Driscoll M. Human DNA damage response and repair deficiency syndromes: linking genomic instability and cell cycle checkpoint proficiency. DNA Repair (Amst) 2009;8:1139–1152. - PubMed
-
- Weinert T.A., Hartwell L.H. The RAD9 gene controls the cell cycle response to DNA damage in Saccharomyces cerevisiae. Science. 1988;241:317–322. - PubMed
-
- Soulier J., Lowndes N.F. The BRCT domain of the S. cerevisiae checkpoint protein Rad9 mediates a Rad9-Rad9 interaction after DNA damage. Curr. Biol. 1999;9:551–554. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

