Bortezomib enhances cancer cell death by blocking the autophagic flux through stimulating ERK phosphorylation
- PMID: 25375375
- PMCID: PMC4260726
- DOI: 10.1038/cddis.2014.468
Bortezomib enhances cancer cell death by blocking the autophagic flux through stimulating ERK phosphorylation
Abstract
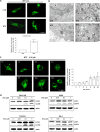
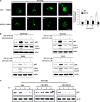
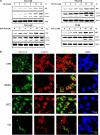
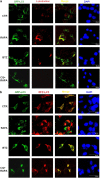
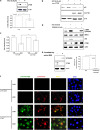
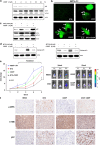
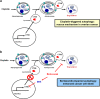
The antitumor activity of an inhibitor of 26S proteasome bortezomib (Velcade) has been observed in various malignancies, including colon cancer, prostate cancer, breast cancer, and ovarian cancer. Bortezomib has been proposed to stimulate autophagy, but scientific observations did not always support this. Interactions between ERK activity and autophagy are complex and not completely clear. Autophagy proteins have recently been shown to regulate the functions of ERK, and ERK activation has been found to induce autophagy. On the other hand, sustained activation of ERK has also been shown to inhibit the maturation step of the autophagy process. In this study, we sought to identify the mechanism of autophagy regulation in cancer cells treated with bortezomib. Our results indicate that bortezomib blocked the autophagic flux without inhibiting the fusion of the autophagosome and lysosome. In ovarian cancer, as well as endometrial cancer and hepatocellular carcinoma cells, bortezomib inhibited protein degradation in lysosomes by suppressing cathepsins, which requires the participation of ERK phosphorylation, but not JNK or p38. Our findings that ERK phosphorylation reduced cathepsins further explain how ERK phosphorylation inhibits the autophagic flux. In conclusion, bortezomib may induce ERK phosphorylation to suppress cathepsin B and inhibit the catalytic process of autophagy in ovarian cancer and other solid tumors. The inhibition of cisplatin-induced autophagy by bortezomib can enhance chemotherapy efficacy in ovarian cancer. As we also found that bortezomib blocks the autophagic flux in other cancers, the synergistic cytotoxic effect of bortezomib by abolishing chemotherapy-related autophagy may help us develop strategies of combination therapies for multiple cancers.
Figures
Similar articles
-
Bortezomib induces apoptosis and autophagy in osteosarcoma cells through mitogen-activated protein kinase pathway in vitro.J Int Med Res. 2013 Oct;41(5):1505-19. doi: 10.1177/0300060513490618. Epub 2013 Aug 23. J Int Med Res. 2013. PMID: 23975859
-
Investigation of the eIF2alpha phosphorylation mechanism in response to proteasome inhibition in melanoma and breast cancer cells.Mol Biol (Mosk). 2010 Sep-Oct;44(5):859-66. Mol Biol (Mosk). 2010. PMID: 21090173
-
ARHI (DIRAS3)-mediated autophagy-associated cell death enhances chemosensitivity to cisplatin in ovarian cancer cell lines and xenografts.Cell Death Dis. 2015 Aug 6;6(8):e1836. doi: 10.1038/cddis.2015.208. Cell Death Dis. 2015. PMID: 26247722 Free PMC article.
-
You eat what you are: autophagy inhibition as a therapeutic strategy in leukemia.Leukemia. 2015 Mar;29(3):517-25. doi: 10.1038/leu.2014.349. Epub 2014 Nov 26. Leukemia. 2015. PMID: 25541151 Free PMC article. Review.
-
Molecular mechanisms for synergistic effect of proteasome inhibitors with platinum-based therapy in solid tumors.Taiwan J Obstet Gynecol. 2016 Feb;55(1):3-8. doi: 10.1016/j.tjog.2015.12.004. Taiwan J Obstet Gynecol. 2016. PMID: 26927239 Review.
Cited by
-
Withaferin A and Celastrol Overwhelm Proteostasis.Int J Mol Sci. 2023 Dec 27;25(1):367. doi: 10.3390/ijms25010367. Int J Mol Sci. 2023. PMID: 38203539 Free PMC article. Review.
-
Lovastatin reduces PEL cell survival by phosphorylating ERK1/2 that blocks the autophagic flux and engages a cross-talk with p53 to activate p21.IUBMB Life. 2021 Jul;73(7):968-977. doi: 10.1002/iub.2503. Epub 2021 May 21. IUBMB Life. 2021. PMID: 33987937 Free PMC article.
-
Apoptosis-related gene expression can predict the response of ovarian cancer cell lines to treatment with recombinant human TRAIL alone or combined with cisplatin.Clinics (Sao Paulo). 2020;75:e1492. doi: 10.6061/clinics/2020/e1492. Epub 2020 Mar 13. Clinics (Sao Paulo). 2020. PMID: 32187278 Free PMC article.
-
Phospholipid scramblase 1 as a critical node at the crossroad between autophagy and apoptosis in mantle cell lymphoma.Onco_target. 2016 Jul 5;7(27):41913-41928. doi: 10.18632/onco_target.9630. Onco_target. 2016. PMID: 27248824 Free PMC article.
-
Erianin suppresses hepatocellular carcinoma cells through down-regulation of PI3K/AKT, p38 and ERK MAPK signaling pathways.Biosci Rep. 2020 Jul 31;40(7):BSR20193137. doi: 10.1042/BSR20193137. Biosci Rep. 2020. PMID: 32677672 Free PMC article.
References
-
- Siegel R, Naishadham D, Jemal A. Cancer statistics, 2013. CA Cancer J Clin. 2013;63:11–30. - PubMed
-
- Katsumata N, Yasuda M, Takahashi F, Isonishi S, Jobo T, Aoki D, et al. Dose-dense paclitaxel once a week in combination with carboplatin every 3 weeks for advanced ovarian cancer: a phase 3, open-label, randomised controlled trial. Lancet. 2009;374:1331–1338. - PubMed
-
- Burger RA, Brady MF, Bookman MA, Fleming GF, Monk BJ, Huang H, et al. Incorporation of bevacizumab in the primary treatment of ovarian cancer. N Engl J Med. 2011;365:2473–2483. - PubMed
-
- Perren TJ, Swart AM, Pfisterer J, Ledermann JA, Pujade-Lauraine E, Kristensen G, et al. A phase 3 trial of bevacizumab in ovarian cancer. N Engl J Med. 2011;365:2484–2496. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

