γ-Butyrobetaine is a proatherogenic intermediate in gut microbial metabolism of L-carnitine to TMAO
- PMID: 25440057
- PMCID: PMC4255476
- DOI: 10.1016/j.cmet.2014.10.006
γ-Butyrobetaine is a proatherogenic intermediate in gut microbial metabolism of L-carnitine to TMAO
Abstract
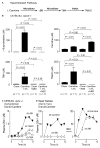
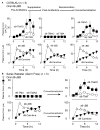
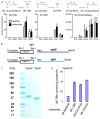
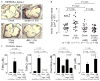
L-carnitine, a nutrient in red meat, was recently reported to accelerate atherosclerosis via a metaorganismal pathway involving gut microbial trimethylamine (TMA) formation and host hepatic conversion into trimethylamine-N-oxide (TMAO). Herein, we show that following L-carnitine ingestion, γ-butyrobetaine (γBB) is produced as an intermediary metabolite by gut microbes at a site anatomically proximal to and at a rate ∼1,000-fold higher than the formation of TMA. Moreover, we show that γBB is the major gut microbial metabolite formed from dietary L-carnitine in mice, is converted into TMA and TMAO in a gut microbiota-dependent manner (like dietary L-carnitine), and accelerates atherosclerosis. Gut microbial composition and functional metabolic studies reveal that distinct taxa are associated with the production of γBB or TMA/TMAO from dietary L-carnitine. Moreover, despite their close structural similarity, chronic dietary exposure to L-carnitine or γBB promotes development of functionally distinct microbial communities optimized for the metabolism of L-carnitine or γBB, respectively.
Copyright © 2014 Elsevier Inc. All rights reserved.
Conflict of interest statement
Drs. Wang and Levison are named as co-inventors on pending patents held by the Cleveland Clinic relating to cardiovascular diagnostics. Dr. Tang received research grant support from Abbott Laboratories, and served as consultants for Medtronic Inc and St. Jude Medical. Drs. Hazen and Smith are named as co-inventors on pending and issued patents held by the Cleveland Clinic relating to cardiovascular diagnostics and therapeutics. Dr. Smith reports he has been paid as a consultant by Esperion, and has the right to recieve royalty payments for inventions from Cleveland Heart Lab and Esperion. Dr. Hazen reports he has been paid as a consultant or speaker by the following companies: Cleveland Heart Lab, Inc., Esperion, Liposciences Inc., Merck & Co., Inc., Pfizer Inc., and Proctor & Gamble. Dr. Hazen reports he has received research funds from Abbott, Cleveland Heart Lab, Liposciences, Inc., Proctor & Gamble, and Takeda. Dr. Hazen has the right to receive royalty payments for inventions or discoveries related to cardiovascular diagnostics and therapeutics from Cleveland Heart Lab, Inc., Esperion, Frantz Biomarkers, and Liposciences, Inc.
Figures


Comment in
-
Mammalian-microbial cometabolism of L-carnitine in the context of atherosclerosis.Cell Metab. 2014 Nov 4;20(5):699-700. doi: 10.1016/j.cmet.2014.10.014. Epub 2014 Nov 4. Cell Metab. 2014. PMID: 25440049
Similar articles
-
l-Carnitine in omnivorous diets induces an atherogenic gut microbial pathway in humans.J Clin Invest. 2019 Jan 2;129(1):373-387. doi: 10.1172/JCI94601. Epub 2018 Dec 10. J Clin Invest. 2019. PMID: 30530985 Free PMC article. Clinical Trial.
-
The microbial gbu gene cluster links cardiovascular disease risk associated with red meat consumption to microbiota L-carnitine catabolism.Nat Microbiol. 2022 Jan;7(1):73-86. doi: 10.1038/s41564-021-01010-x. Epub 2021 Dec 23. Nat Microbiol. 2022. PMID: 34949826 Free PMC article.
-
Major Increase in Microbiota-Dependent Proatherogenic Metabolite TMAO One Year After Bariatric Surgery.Metab Syndr Relat Disord. 2016 May;14(4):197-201. doi: 10.1089/met.2015.0120. Epub 2016 Jan 26. Metab Syndr Relat Disord. 2016. PMID: 27081744 Free PMC article.
-
Metaorganismal nutrient metabolism as a basis of cardiovascular disease.Curr Opin Lipidol. 2014 Feb;25(1):48-53. doi: 10.1097/MOL.0000000000000036. Curr Opin Lipidol. 2014. PMID: 24362355 Free PMC article. Review.
-
Dietary bioactive ingredients to modulate the gut microbiota-derived metabolite TMAO. New opportunities for functional food development.Food Funct. 2020 Aug 1;11(8):6745-6776. doi: 10.1039/d0fo01237h. Epub 2020 Jul 20. Food Funct. 2020. PMID: 32686802 Review.
Cited by
-
Listening to Our Gut: Contribution of Gut Microbiota and Cardiovascular Risk in Diabetes Pathogenesis.Curr Diab Rep. 2015 Sep;15(9):63. doi: 10.1007/s11892-015-0634-1. Curr Diab Rep. 2015. PMID: 26208694 Free PMC article. Review.
-
Immune and inflammatory mechanisms of abdominal aortic aneurysm.Front Immunol. 2022 Oct 5;13:989933. doi: 10.3389/fimmu.2022.989933. eCollection 2022. Front Immunol. 2022. PMID: 36275758 Free PMC article. Review.
-
Microbiome therapeutics - Advances and challenges.Adv Drug Deliv Rev. 2016 Oct 1;105(Pt A):44-54. doi: 10.1016/j.addr.2016.04.032. Epub 2016 May 5. Adv Drug Deliv Rev. 2016. PMID: 27158095 Free PMC article. Review.
-
Uraemic solutes as therapeutic _targets in CKD-associated cardiovascular disease.Nat Rev Nephrol. 2021 Jun;17(6):402-416. doi: 10.1038/s41581-021-00408-4. Epub 2021 Mar 23. Nat Rev Nephrol. 2021. PMID: 33758363 Review.
-
Role of Gut Microbial Metabolites in Cardiovascular Diseases-Current Insights and the Road Ahead.Int J Mol Sci. 2024 Sep 23;25(18):10208. doi: 10.3390/ijms251810208. Int J Mol Sci. 2024. PMID: 39337693 Free PMC article. Review.
References
-
- Aurich H. Composition of the amino acid pool of Neurospora in deficiency of growth substance. Acta biologica et medica Germanica. 1966;16:123–134. - PubMed
-
- Bennett BJ, de Aguiar Vallim TQ, Wang Z, Shih DM, Meng Y, Gregory J, Allayee H, Lee R, Graham M, Crooke R, Edwards PA, Hazen SL, Lusis AJ. Trimethylamine-N-oxide, a metabolite associated with atherosclerosis, exhibits complex genetic and dietary regulation. Cell metabolism. 2013;17:49–60. - PMC - PubMed
-
- Bremer J. Carnitine--metabolism and functions. Physiological Reviews. 1983;63:1420–1480. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

