Crystal structure of N'-hy-droxy-pyrimidine-2-carboximidamide
- PMID: 25484698
- PMCID: PMC4257198
- DOI: 10.1107/S1600536814020285
Crystal structure of N'-hy-droxy-pyrimidine-2-carboximidamide
Abstract
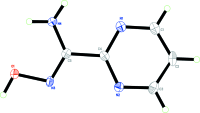
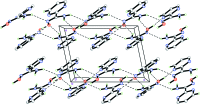
The title compound, C5H6N4O, is approximately planar, with an angle of 11.04 (15)° between the planes of the pyrimidine ring and the non-H atoms of the carboximidamide unit. The mol-ecule adopts an E configuration about the C=N double bond. In the crystal, adjacent mol-ecules are linked by pairs of N-H⋯O hydrogen bonds, forming inversion dimers with an R 2 (2)(10) ring motif. The dimers are further linked via N-H⋯N and O-H⋯N hydrogen bonds into a sheet structure parallel to the ac plane. The crystal structure also features N-H⋯O and weak C-H⋯O hydrogen bonds and offset π-π stacking inter-actions between adjacent pyrimidine rings [centroid-centroid distance = 3.622 (1) Å].
Keywords: biological activity; crystal structure; hydrogen bonding; non-covalent interactions; pyrimidine-2-carboximidamide; π–π stacking interactions.
Figures
Similar articles
-
Crystal structure of 2-hy-droxy-imino-2-(pyridin-2-yl)-N'-[1-(pyridin-2-yl)ethyl-idene]acetohydrazide.Acta Crystallogr Sect E Struct Rep Online. 2014 Nov 29;70(Pt 12):584-6. doi: 10.1107/S1600536814025793. eCollection 2014 Dec 1. Acta Crystallogr Sect E Struct Rep Online. 2014. PMID: 25552998 Free PMC article.
-
Crystal structures of (E)-N'-(2-hy-droxy-5-methyl-benzyl-idene)isonicotinohydrazide and (E)-N'-(5-fluoro-2-hy-droxy-benzyl-idene)isonicotinohydrazide.Acta Crystallogr E Crystallogr Commun. 2016 Jun 17;72(Pt 7):980-3. doi: 10.1107/S2056989016009762. eCollection 2016 Jul 1. Acta Crystallogr E Crystallogr Commun. 2016. PMID: 27555945 Free PMC article.
-
Crystal structures of 3-chloro-2-nitro-benzoic acid with quinoline derivatives: 3-chloro-2-nitro-benzoic acid-5-nitro-quinoline (1/1), 3-chloro-2-nitro-benzoic acid-6-nitro-quinoline (1/1) and 8-hy-droxy-quinolinium 3-chloro-2-nitro-benzoate.Acta Crystallogr E Crystallogr Commun. 2019 Sep 27;75(Pt 10):1552-1557. doi: 10.1107/S2056989019012799. eCollection 2019 Oct 1. Acta Crystallogr E Crystallogr Commun. 2019. PMID: 31636993 Free PMC article.
-
Crystal structure of (E)-N'-(5-bromo-2-hy-droxy-benzyl-idene)nicotinohydrazide monohydrate.Acta Crystallogr E Crystallogr Commun. 2015 Jun 3;71(Pt 7):734-6. doi: 10.1107/S2056989015009627. eCollection 2015 Jul 1. Acta Crystallogr E Crystallogr Commun. 2015. PMID: 26279854 Free PMC article.
-
(E)-N'-(3-Bromo-5-chloro-2-hy-droxy-benzyl-idene)nicotinohydrazide.Acta Crystallogr Sect E Struct Rep Online. 2011 Oct 1;67(Pt 10):o2716. doi: 10.1107/S1600536811038268. Epub 2011 Sep 30. Acta Crystallogr Sect E Struct Rep Online. 2011. PMID: 22065830 Free PMC article.
References
-
- Aakeroy, C. B. & Seddon, K. R. (1993). Chem. Soc. Rev. 22, 397–407.
-
- Bruker (2009). SADABS, APEX2 and SAINT Bruker AXS Inc., Madison, Wisconsin, USA.
-
- Desiraju, G. R. (2007). Angew. Chem. Int. Ed. 46, 8342–8356. - PubMed
-
- Kundu, M., Singh, B., Ghosh, T., Maiti, B. C. & Maity, T. K. (2012). Indian J. Chem. Sect. B, 51, 493–497.
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous
