Multicenter Phase II Trial of Temsirolimus and Bevacizumab in Pancreatic Neuroendocrine Tumors
- PMID: 25488966
- PMCID: PMC4417726
- DOI: 10.1200/JCO.2014.56.2082
Multicenter Phase II Trial of Temsirolimus and Bevacizumab in Pancreatic Neuroendocrine Tumors
Abstract
Purpose: There are few effective therapies for pancreatic neuroendocrine tumors (PNETs). Recent placebo-controlled phase III trials of the mammalian _target of rapamycin (mTOR) inhibitor everolimus and the vascular endothelial growth factor (VEGF)/platelet-derived growth factor receptor inhibitor sunitinib have noted improved progression-free survival (PFS). Preclinical studies have suggested enhanced antitumor effects with combined mTOR and VEGF pathway-_targeted therapy. We conducted a clinical trial to evaluate combination therapy against these _targets in PNETs.
Patients and methods: We conducted a two-stage single-arm phase II trial of the mTOR inhibitor temsirolimus 25 mg intravenously (IV) once per week and the VEGF-A monoclonal antibody bevacizumab 10 mg/kg IV once every 2 weeks in patients with well or moderately differentiated PNETs and progressive disease by RECIST within 7 months of study entry. Coprimary end points were tumor response rate and 6-month PFS.
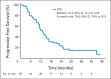
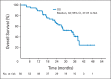
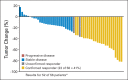
Results: A total of 58 patients were enrolled, and 56 patients were eligible for response assessment. Confirmed response rate (RR) was 41% (23 of 56 patients). PFS at 6 months was 79% (44 of 56). Median PFS was 13.2 months (95% CI, 11.2 to 16.6). Median overall survival was 34 months (95% CI, 27.1 to not reached). For evaluable patients, the most common grade 3 to 4 adverse events attributed to therapy were hypertension (21%), fatigue (16%), lymphopenia (14%), and hyperglycemia (14%).
Conclusion: The combination of temsirolimus and bevacizumab had substantial activity and reasonable tolerability in a multicenter phase II trial, with RR of 41%, well in excess of single _targeted agents in patients with progressive PNETs. Six-month PFS was a notable 79% in a population of patients with disease progression by RECIST criteria within 7 months of study entry. On the basis of this trial, continued evaluation of combination mTOR and VEGF pathway inhibitors is warranted.
Trial registration: ClinicalTrials.gov NCT01010126.
© 2014 by American Society of Clinical Oncology.
Conflict of interest statement
Authors' disclosures of potential conflicts of interest and author contributions are found at the end of this article.
Figures
Comment in
-
Combined mammalian _target of rapamycin and vascular endothelial growth factor pathway inhibition in pancreatic neuroendocrine tumors: more than the sum of its parts?J Clin Oncol. 2015 May 10;33(14):1523-6. doi: 10.1200/JCO.2014.58.6974. Epub 2015 Feb 2. J Clin Oncol. 2015. PMID: 25646187 No abstract available.
Similar articles
-
BEST: A Randomized Phase II Study of Vascular Endothelial Growth Factor, RAF Kinase, and Mammalian _target of Rapamycin Combination _targeted Therapy With Bevacizumab, Sorafenib, and Temsirolimus in Advanced Renal Cell Carcinoma--A Trial of the ECOG-ACRIN Cancer Research Group (E2804).J Clin Oncol. 2015 Jul 20;33(21):2384-91. doi: 10.1200/JCO.2015.60.9727. Epub 2015 Jun 15. J Clin Oncol. 2015. PMID: 26077237 Free PMC article. Clinical Trial.
-
Safety and activity of temsirolimus and bevacizumab in patients with advanced renal cell carcinoma previously treated with tyrosine kinase inhibitors: a phase 2 consortium study.Cancer Chemother Pharmacol. 2015 Mar;75(3):485-93. doi: 10.1007/s00280-014-2668-5. Epub 2015 Jan 3. Cancer Chemother Pharmacol. 2015. PMID: 25556030 Free PMC article. Clinical Trial.
-
Phase I/II study evaluating the safety and clinical efficacy of temsirolimus and bevacizumab in patients with chemotherapy refractory metastatic castration-resistant prostate cancer.Invest New Drugs. 2019 Apr;37(2):331-337. doi: 10.1007/s10637-018-0687-5. Epub 2018 Nov 7. Invest New Drugs. 2019. PMID: 30402678 Clinical Trial.
-
Resistance to _targeted therapies in pancreatic neuroendocrine tumors (PNETs): molecular basis, preclinical data, and counteracting strategies._target Oncol. 2012 Sep;7(3):173-81. doi: 10.1007/s11523-012-0229-6. Epub 2012 Aug 25. _target Oncol. 2012. PMID: 22923165 Review.
-
PI3K/Akt/mTOR pathway inhibitors in the therapy of pancreatic neuroendocrine tumors.Cancer Lett. 2013 Jul 10;335(1):1-8. doi: 10.1016/j.canlet.2013.02.016. Epub 2013 Feb 16. Cancer Lett. 2013. PMID: 23419523 Review.
Cited by
-
An Overview on the Sequential Treatment of Pancreatic Neuroendocrine Tumors (pNETs).Rare Cancers Ther. 2015;3(1):13-33. doi: 10.1007/s40487-015-0007-6. Epub 2015 Jul 11. Rare Cancers Ther. 2015. PMID: 27182476 Free PMC article. Review.
-
Pancreatic neuroendocrine tumors: the basics, the gray zone, and the _target.F1000Res. 2017 May 10;6:663. doi: 10.12688/f1000research.10188.1. eCollection 2017. F1000Res. 2017. PMID: 28529726 Free PMC article. Review.
-
Phase III Prospective Randomized Comparison Trial of Depot Octreotide Plus Interferon Alfa-2b Versus Depot Octreotide Plus Bevacizumab in Patients With Advanced Carcinoid Tumors: SWOG S0518.J Clin Oncol. 2017 May 20;35(15):1695-1703. doi: 10.1200/JCO.2016.70.4072. Epub 2017 Apr 6. J Clin Oncol. 2017. PMID: 28384065 Free PMC article. Clinical Trial.
-
Molecular Signatures and Their Clinical Utility in Pancreatic Neuroendocrine Tumors.Front Endocrinol (Lausanne). 2021 Jan 18;11:575620. doi: 10.3389/fendo.2020.575620. eCollection 2020. Front Endocrinol (Lausanne). 2021. PMID: 33537001 Free PMC article. Review.
-
Vorolanib (X-82), an oral anti-VEGFR/PDGFR/CSF1R tyrosine kinase inhibitor, with everolimus in solid tumors: results of a phase I study.Invest New Drugs. 2021 Oct;39(5):1298-1305. doi: 10.1007/s10637-021-01093-7. Epub 2021 Mar 18. Invest New Drugs. 2021. PMID: 33738668 Clinical Trial.
References
-
- Moertel CG, Lefkopoulo M, Lipsitz S, et al. Streptozocin-doxorubicin, streptozocin-flourouracil or chlorozotocin in the treatment of advanced islet-cell carcinoma. N Engl J Med. 1992;326:519–523. - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous