Proteins in aggregates functionally impact multiple neurodegenerative disease models by forming proteasome-blocking complexes
- PMID: 25510159
- PMCID: PMC4326912
- DOI: 10.1111/acel.12296
Proteins in aggregates functionally impact multiple neurodegenerative disease models by forming proteasome-blocking complexes
Abstract
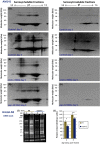
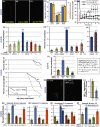
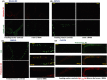
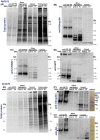
Age-dependent neurodegenerative diseases progressively form aggregates containing both shared components (e.g., TDP-43, phosphorylated tau) and proteins specific to each disease. We investigated whether diverse neuropathies might have additional aggregation-prone proteins in common, discoverable by proteomics. Caenorhabditis elegans expressing unc-54p/Q40::YFP, a model of polyglutamine array diseases such as Huntington's, accrues aggregates in muscle 2-6 days posthatch. These foci, isolated on antibody-coupled magnetic beads, were characterized by high-resolution mass spectrometry. Three Q40::YFP-associated proteins were inferred to promote aggregation and cytotoxicity, traits reduced or delayed by their RNA interference knockdown. These RNAi treatments also retarded aggregation/cytotoxicity in Alzheimer's disease models, nematodes with muscle or pan-neuronal Aβ₁₋₄₂ expression and behavioral phenotypes. The most abundant aggregated proteins are glutamine/asparagine-rich, favoring hydrophobic interactions with other random-coil domains. A particularly potent modulator of aggregation, CRAM-1/HYPK, contributed < 1% of protein aggregate peptides, yet its knockdown reduced Q40::YFP aggregates 72-86% (P < 10(-6) ). In worms expressing Aβ₁₋₄₂, knockdown of cram-1 reduced β-amyloid 60% (P < 0.002) and slowed age-dependent paralysis > 30% (P < 10(-6)). In wild-type worms, cram-1 knockdown reduced aggregation and extended lifespan, but impaired early reproduction. Protection against seeded aggregates requires proteasome function, implying that normal CRAM-1 levels promote aggregation by interfering with proteasomal degradation of misfolded proteins. Molecular dynamic modeling predicts spontaneous and stable interactions of CRAM-1 (or human orthologs) with ubiquitin, and we verified that CRAM-1 reduces degradation of a tagged-ubiquitin reporter. We propose that CRAM-1 exemplifies a class of primitive chaperones that are initially protective and highly beneficial for early reproduction, but ultimately impair aggregate clearance and limit longevity.
Keywords: (protein) aggregation; Alzheimer (disease); C. elegans; Huntington (disease); neurodegeneration; proteasome.
© 2014 The Authors. Aging Cell published by the Anatomical Society and John Wiley & Sons Ltd.
Figures

Similar articles
-
Proteins that mediate protein aggregation and cytotoxicity distinguish Alzheimer's hippocampus from normal controls.Aging Cell. 2016 Oct;15(5):924-39. doi: 10.1111/acel.12501. Epub 2016 Jul 23. Aging Cell. 2016. PMID: 27448508 Free PMC article.
-
Structural insights into pro-aggregation effects of C. elegans CRAM-1 and its human ortholog SERF2.Sci Rep. 2018 Oct 5;8(1):14891. doi: 10.1038/s41598-018-33143-1. Sci Rep. 2018. PMID: 30291272 Free PMC article.
-
Amyloid-beta (Aβ₁₋₄₂)-induced paralysis in Caenorhabditis elegans is inhibited by the polyphenol quercetin through activation of protein degradation pathways.Mol Nutr Food Res. 2014 Oct;58(10):1931-40. doi: 10.1002/mnfr.201400014. Epub 2014 Jul 28. Mol Nutr Food Res. 2014. PMID: 25066301
-
Ubiquitin proteasome system as a pharmacological _target in neurodegeneration.Expert Rev Neurother. 2006 Sep;6(9):1337-47. doi: 10.1586/14737175.6.9.1337. Expert Rev Neurother. 2006. PMID: 17009921 Review.
-
Degradation of misfolded proteins in neurodegenerative diseases: therapeutic _targets and strategies.Exp Mol Med. 2015 Mar 13;47(3):e147. doi: 10.1038/emm.2014.117. Exp Mol Med. 2015. PMID: 25766616 Free PMC article. Review.
Cited by
-
Molecular Insight Into the IRE1α-Mediated Type I Interferon Response Induced by Proteasome Impairment in Myeloid Cells of the Brain.Front Immunol. 2019 Dec 18;10:2900. doi: 10.3389/fimmu.2019.02900. eCollection 2019. Front Immunol. 2019. PMID: 31921161 Free PMC article.
-
ALS-causing mutations in profilin-1 alter its conformational dynamics: A computational approach to explain propensity for aggregation.Sci Rep. 2018 Aug 30;8(1):13102. doi: 10.1038/s41598-018-31199-7. Sci Rep. 2018. PMID: 30166578 Free PMC article.
-
An IRES-dependent translation of HYPK mRNA generates a truncated isoform of the protein that lacks the nuclear localization and functional ability.RNA Biol. 2019 Nov;16(11):1604-1621. doi: 10.1080/15476286.2019.1650612. Epub 2019 Aug 9. RNA Biol. 2019. PMID: 31397627 Free PMC article.
-
Novel Contributors and Mechanisms of Cellular Senescence in Hypertension-Associated Premature Vascular Aging.Am J Hypertens. 2019 Jul 17;32(8):709-719. doi: 10.1093/ajh/hpz052. Am J Hypertens. 2019. PMID: 30982879 Free PMC article. Review.
-
Proteins that mediate protein aggregation and cytotoxicity distinguish Alzheimer's hippocampus from normal controls.Aging Cell. 2016 Oct;15(5):924-39. doi: 10.1111/acel.12501. Epub 2016 Jul 23. Aging Cell. 2016. PMID: 27448508 Free PMC article.
References
-
- Arnesen T, Starheim KK, Van DP, Evjenth R, Dinh H, Betts MJ, Ryningen A, Vandekerckhove J, Gevaert K, Anderson D. The chaperone-like protein HYPK acts together with NatA in cotranslational N-terminal acetylation and prevention of Huntingtin aggregation. Mol. Cell. Biol. 2010;30:1898–1909. - PMC - PubMed
-
- Ayyadevara S, Bharill P, Dandapat A, Hu CP, Khaidakov M, Mitra S, Shmookler Reis RJ, Mehta JL. Aspirin inhibits oxidant stress, reduces aging-associated functional declines and extends lifespan of Caenorhabditis elegans. Antioxid. Redox Signal. 2013;18:481–490. - PubMed
-
- Bennett EJ, Shaler TA, Woodman B, Ryu KY, Zaitseva TS, Becker CH, Bates GP, Schulman H, Kopito RR. Global changes to the ubiquitin system in Huntington's disease. Nature. 2007;448:704–708. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

