Resveratrol regulates mitochondrial reactive oxygen species homeostasis through Sirt3 signaling pathway in human vascular endothelial cells
- PMID: 25522270
- PMCID: PMC4454164
- DOI: 10.1038/cddis.2014.530
Resveratrol regulates mitochondrial reactive oxygen species homeostasis through Sirt3 signaling pathway in human vascular endothelial cells
Abstract
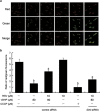
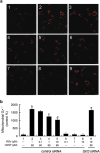
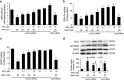
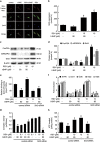
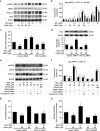
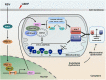
Mitochondrial reactive oxygen species (mtROS) homeostasis plays an essential role in preventing oxidative injury in endothelial cells, an initial step in atherogenesis. Resveratrol (RSV) possesses a variety of cardioprotective activities, however, little is known regarding the effects of RSV on mtROS homeostasis in endothelial cells. Sirt3 is a mitochondrial deacetylase, which plays a key role in mitochondrial bioenergetics and is closely associated with oxidative stress. The goal of the study is to investigate whether RSV could attenuate oxidative injury in endothelial cells via mtROS homeostasis regulation through Sirt3 signaling pathway. We found that pretreatment with RSV suppressed tert-butyl hydroperoxide (t-BHP)-induced oxidative damage in human umbilical vein endothelial cells (HUVECs) by increasing cell viability, inhibiting cell apoptosis, repressing collapse of mitochondrial membrane potential and decreasing mtROS generation. Moreover, the enzymatic activities of isocitrate dehydrogenase 2 (IDH2), glutathione peroxidase (GSH-Px) and manganese superoxide dismutase (SOD2) as well as deacetylation of SOD2 were increased by RSV pretreatment, suggesting RSV notably enhanced mtROS scavenging in t-BHP-induced endothelial cells. Meanwhile, RSV remarkably reduced mtROS generation by promoting Sirt3 enrichment within the mitochondria and subsequent upregulation of forkhead box O3A (FoxO3A)-mediated mitochondria-encoded gene expression of ATP6, CO1, Cytb, ND2 and ND5, thereby leading to increased complex I activity and ATP synthesis. Furthermore, RSV activated the expressions of phosphorylated adenosine monophosphate-activated protein kinase (p-AMPK), peroxisome proliferator-activated receptor gamma coactivator-1α (PGC-1α) and Sirt3, as well as estrogen-related receptor-α (ERRα)-dependent Sirt3 mRNA transcription, which were abolished in the presence of AMPK inhibitor and AMPK, PGC-1α or Sirt3 siRNA transfection, indicating the effects of RSV on mtROS homeostasis regulation were dependent on AMPK-PGC-1α-ERRα-Sirt3 signaling pathway. Our findings indicated a novel mechanism that RSV-attenuated oxidative injury in endothelial cells through the regulation of mtROS homeostasis, which, in part, was mediated through the activation of the Sirt3 signaling pathway.
Figures

Similar articles
-
Resveratrol rescues cadmium-induced mitochondrial injury by enhancing transcriptional regulation of PGC-1α and SOD2 via the Sirt3/FoxO3a pathway in TCMK-1 cells.Biochem Biophys Res Commun. 2017 Apr 22;486(1):198-204. doi: 10.1016/j.bbrc.2017.03.027. Epub 2017 Mar 9. Biochem Biophys Res Commun. 2017. PMID: 28286268
-
Trilobatin Protects Against Oxidative Injury in Neuronal PC12 Cells Through Regulating Mitochondrial ROS Homeostasis Mediated by AMPK/Nrf2/Sirt3 Signaling Pathway.Front Mol Neurosci. 2018 Jul 30;11:267. doi: 10.3389/fnmol.2018.00267. eCollection 2018. Front Mol Neurosci. 2018. PMID: 30104959 Free PMC article.
-
Melatonin-mediated upregulation of Sirt3 attenuates sodium fluoride-induced hepatotoxicity by activating the MT1-PI3K/AKT-PGC-1α signaling pathway.Free Radic Biol Med. 2017 Nov;112:616-630. doi: 10.1016/j.freeradbiomed.2017.09.005. Epub 2017 Sep 11. Free Radic Biol Med. 2017. PMID: 28912098
-
Cytoprotective Effect of the UCP2-SIRT3 Signaling Pathway by Decreasing Mitochondrial Oxidative Stress on Cerebral Ischemia-Reperfusion Injury.Int J Mol Sci. 2017 Jul 24;18(7):1599. doi: 10.3390/ijms18071599. Int J Mol Sci. 2017. PMID: 28737710 Free PMC article. Review.
-
Natural products, PGC-1 α , and Duchenne muscular dystrophy.Acta Pharm Sin B. 2020 May;10(5):734-745. doi: 10.1016/j.apsb.2020.01.001. Epub 2020 Jan 8. Acta Pharm Sin B. 2020. PMID: 32528825 Free PMC article. Review.
Cited by
-
Perampanel attenuates oxidative stress and pyroptosis following subarachnoid hemorrhage via the SIRT3/FOXO3α pathway.Sci Rep. 2023 Dec 3;13(1):21320. doi: 10.1038/s41598-023-48802-1. Sci Rep. 2023. PMID: 38044382 Free PMC article.
-
Sirtuin 3 Deregulation Promotes Pulmonary Fibrosis.J Gerontol A Biol Sci Med Sci. 2017 May 1;72(5):595-602. doi: 10.1093/gerona/glw151. J Gerontol A Biol Sci Med Sci. 2017. PMID: 27522058 Free PMC article.
-
Pterostilbene Attenuates High-Intensity Swimming Exercise-Induced Glucose Absorption Dysfunction Associated with the Inhibition of NLRP3 Inflammasome-Induced IECs Pyroptosis.Nutrients. 2023 Apr 23;15(9):2036. doi: 10.3390/nu15092036. Nutrients. 2023. PMID: 37432144 Free PMC article.
-
Sirt3 suppresses calcium oxalate-induced renal tubular epithelial cell injury via modification of FoxO3a-mediated autophagy.Cell Death Dis. 2019 Jan 15;10(2):34. doi: 10.1038/s41419-018-1169-6. Cell Death Dis. 2019. Retraction in: Cell Death Dis. 2020 Feb 10;11(2):113. doi: 10.1038/s41419-020-2318-2 PMID: 30674870 Free PMC article. Retracted.
-
Combined effect of 17β-estradiol and resveratrol against apoptosis induced by interleukin-1β in rat nucleus pulposus cells via PI3K/Akt/caspase-3 pathway.PeerJ. 2016 Jan 26;4:e1640. doi: 10.7717/peerj.1640. eCollection 2016. PeerJ. 2016. PMID: 26824000 Free PMC article.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

