Intestinal FXR agonism promotes adipose tissue browning and reduces obesity and insulin resistance
- PMID: 25559344
- PMCID: PMC4320010
- DOI: 10.1038/nm.3760
Intestinal FXR agonism promotes adipose tissue browning and reduces obesity and insulin resistance
Abstract
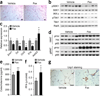
The systemic expression of the bile acid (BA) sensor farnesoid X receptor (FXR) has led to promising new therapies _targeting cholesterol metabolism, triglyceride production, hepatic steatosis and biliary cholestasis. In contrast to systemic therapy, bile acid release during a meal selectively activates intestinal FXR. By mimicking this tissue-selective effect, the gut-restricted FXR agonist fexaramine (Fex) robustly induces enteric fibroblast growth factor 15 (FGF15), leading to alterations in BA composition, but does so without activating FXR _target genes in the liver. However, unlike systemic agonism, we find that Fex reduces diet-induced weight gain, body-wide inflammation and hepatic glucose production, while enhancing thermogenesis and browning of white adipose tissue (WAT). These pronounced metabolic improvements suggest tissue-restricted FXR activation as a new approach in the treatment of obesity and metabolic syndrome.
Figures
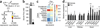

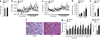

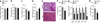
Comment in
-
Obesity: Gut-specific FXR agonism.Nat Rev Endocrinol. 2015 Mar;11(3):131. doi: 10.1038/nrendo.2015.4. Epub 2015 Jan 27. Nat Rev Endocrinol. 2015. PMID: 25623122 No abstract available.
-
Obesity and diabetes: FXR and JAK step up to BAT.Nat Rev Drug Discov. 2015 Feb;14(2):91. doi: 10.1038/nrd4543. Nat Rev Drug Discov. 2015. PMID: 25633788 No abstract available.
Similar articles
-
Obesity: Gut-specific FXR agonism.Nat Rev Endocrinol. 2015 Mar;11(3):131. doi: 10.1038/nrendo.2015.4. Epub 2015 Jan 27. Nat Rev Endocrinol. 2015. PMID: 25623122 No abstract available.
-
Obesity and diabetes: FXR and JAK step up to BAT.Nat Rev Drug Discov. 2015 Feb;14(2):91. doi: 10.1038/nrd4543. Nat Rev Drug Discov. 2015. PMID: 25633788 No abstract available.
-
Intestine farnesoid X receptor agonist and the gut microbiota activate G-protein bile acid receptor-1 signaling to improve metabolism.Hepatology. 2018 Oct;68(4):1574-1588. doi: 10.1002/hep.29857. Epub 2018 May 21. Hepatology. 2018. PMID: 29486523 Free PMC article.
-
Nuclear receptor FXR, bile acids and liver damage: Introducing the progressive familial intrahepatic cholestasis with FXR mutations.Biochim Biophys Acta Mol Basis Dis. 2018 Apr;1864(4 Pt B):1308-1318. doi: 10.1016/j.bbadis.2017.09.019. Epub 2017 Sep 29. Biochim Biophys Acta Mol Basis Dis. 2018. PMID: 28965883 Review.
-
Intestine-specific FXR agonists as potential therapeutic agents for colorectal cancer.Biochem Pharmacol. 2021 Apr;186:114430. doi: 10.1016/j.bcp.2021.114430. Epub 2021 Feb 6. Biochem Pharmacol. 2021. PMID: 33556338 Review.
Cited by
-
Role of Bile Acids in the Regulation of Food Intake, and Their Dysregulation in Metabolic Disease.Nutrients. 2021 Mar 28;13(4):1104. doi: 10.3390/nu13041104. Nutrients. 2021. PMID: 33800566 Free PMC article. Review.
-
Microbial metabolites as a way to provide crosstalk between gut and liver.Obstet Med. 2024 Sep;17(3):168-174. doi: 10.1177/1753495X241258383. Epub 2024 Jun 5. Obstet Med. 2024. PMID: 39262911 Review.
-
Western diet induces Paneth cell defects through microbiome alterations and farnesoid X receptor and type I interferon activation.Cell Host Microbe. 2021 Jun 9;29(6):988-1001.e6. doi: 10.1016/j.chom.2021.04.004. Epub 2021 May 18. Cell Host Microbe. 2021. PMID: 34010595 Free PMC article.
-
A High-Fat Diet Increases Gut Microbiota Biodiversity and Energy Expenditure Due to Nutrient Difference.Nutrients. 2020 Oct 20;12(10):3197. doi: 10.3390/nu12103197. Nutrients. 2020. PMID: 33092019 Free PMC article.
-
The Interaction of Gut Microbiota and Heart Failure with Preserved Ejection Fraction: From Mechanism to Potential Therapies.Biomedicines. 2023 Feb 2;11(2):442. doi: 10.3390/biomedicines11020442. Biomedicines. 2023. PMID: 36830978 Free PMC article. Review.
References
-
- Forman BM, et al. Identification of a nuclear receptor that is activated by farnesol metabolites. Cell. 1995;81:687–693. - PubMed
-
- Lee FY, Lee H, Hubbert ML, Edwards PA, Zhang Y. FXR, a multipurpose nuclear receptor. Trends Biochem. Sci. 2006;31:572–580. - PubMed
-
- Repa JJ, et al. Regulation of absorption and ABC1-mediated efflux of cholesterol by RXR heterodimers. Science. 2000;289:1524–1529. - PubMed
-
- Zollner G, et al. Role of nuclear bile acid receptor, FXR, in adaptive ABC transporter regulation by cholic and ursodeoxycholic acid in mouse liver, kidney and intestine. J. Hepatol. 2003;39:480–488. - PubMed
MeSH terms
Substances
Associated data
Grants and funding
- P01 HL088093/HL/NHLBI NIH HHS/United States
- R01 HL105278/HL/NHLBI NIH HHS/United States
- R24 DK090962/DK/NIDDK NIH HHS/United States
- P01 DK074868/DK/NIDDK NIH HHS/United States
- P42 ES010337/ES/NIEHS NIH HHS/United States
- R01 DK033651/DK/NIDDK NIH HHS/United States
- P30 DK063491/DK/NIDDK NIH HHS/United States
- T32 DK007494/DK/NIDDK NIH HHS/United States
- R01 DK061618/DK/NIDDK NIH HHS/United States
- P01 DK054441/DK/NIDDK NIH HHS/United States
- R37 DK057978/DK/NIDDK NIH HHS/United States
- R01 DK060597/DK/NIDDK NIH HHS/United States
- HHMI/Howard Hughes Medical Institute/United States
- U54 HD012303/HD/NICHD NIH HHS/United States
- R37 DK033651/DK/NIDDK NIH HHS/United States
- R01 DK060591/DK/NIDDK NIH HHS/United States
- R01 DK057978/DK/NIDDK NIH HHS/United States
- P30 CA014195/CA/NCI NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

