Heterozygous deletion of ATG5 in Apc(Min/+) mice promotes intestinal adenoma growth and enhances the antitumor efficacy of interferon-gamma
- PMID: 25695667
- PMCID: PMC4622965
- DOI: 10.1080/15384047.2014.1002331
Heterozygous deletion of ATG5 in Apc(Min/+) mice promotes intestinal adenoma growth and enhances the antitumor efficacy of interferon-gamma
Abstract
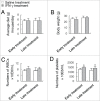
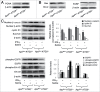
Autophagy related gene 5 (ATG5) was lost in 23% of the patients with colorectal cancer (CRC) and the role of loss of ATG5 in the pathogenesis of CRC remains unclear. Knockdown of ATG5 in cancer cells enhances the antitumor efficacy of lots of chemotherapeutic agents. However, there is still no animal model to validate these in vitro observations in vivo. In this study, we found that heterozygous deletion of ATG5 in Apc(Min/+) mice increased the number and size of adenomas as compared with those in Apc(Min/+)ATG5(+/+) mice. To investigate whether ATG5 deficiency could sensitize tumors to chemotherapies, we compared the antitumor effects of Interferon-gamma (IFN-γ) between Apc(Min/+)ATG5(+/+) and Apc(Min/+)ATG5(+/-) mice, as IFN-γ is a potential tumor suppressor for CRC and has been used clinically as an efficient adjuvant to chemotherapy of cancer. We revealed that heterozygous deletion of ATG5 significantly enhanced the antitumor efficacy of IFN-γ. Early treatment of Apc(Min/+)ATG5(+/-) mice with IFN-γ decreased tumor incidence rate to 16.7% and reduced the number of adenomas by 95.5% and late treatment led to regression of tumor. Moreover, IFN-γ treatment did not cause any evident toxic reaction. Mechanistic analysis revealed that heterozygous deletion of ATG5 activated EGFR/ERK1/2 and Wnt/β-catenin pathways in adenomas of Apc(Min/+) mice and enhanced the effects of IFN-γ-dependent inhibition of these 2 pathways. Our results demonstrate that ATG5 plays important roles in intestinal tumor growth and combination of IFN-γ and ATG5 deficiency or ATG5-_targeted inhibition is a promising strategy for prevention and treatment of CRC.
Keywords: 5-FU, 5-fluorouracil; ATG5; ATG5, autophagy related gene 5; Apc, adenomatous polyposis coli; ApcMin/+ mouse; CRC, colorectal cancer; EGFR, epidermal growth factor receptor; Erk, extracellular signal-regulated kinase; IFN-γ; IFN-γ, Interferon-gamma; LC3, microtubule-associated protein 1 light chain 3; PCNA, proliferating cell nuclear antigen; colorectal cancer; heterozygous deletion; intestinal adenoma; siRNAs, small interfering RNAs.
Figures


Similar articles
-
Mutated K-ras(Asp12) promotes tumourigenesis in Apc(Min) mice more in the large than the small intestines, with synergistic effects between K-ras and Wnt pathways.Int J Exp Pathol. 2009 Oct;90(5):558-74. doi: 10.1111/j.1365-2613.2009.00667.x. Int J Exp Pathol. 2009. PMID: 19765110 Free PMC article.
-
Haploinsufficiency of Krüppel-like factor 4 promotes adenomatous polyposis coli dependent intestinal tumorigenesis.Cancer Res. 2007 Aug 1;67(15):7147-54. doi: 10.1158/0008-5472.CAN-07-1302. Cancer Res. 2007. PMID: 17671182 Free PMC article.
-
Deficiency of interferon-gamma or its receptor promotes colorectal cancer development.J Interferon Cytokine Res. 2015 Apr;35(4):273-80. doi: 10.1089/jir.2014.0132. Epub 2014 Nov 10. J Interferon Cytokine Res. 2015. PMID: 25383957
-
The intestinal epithelium and its neoplasms: genetic, cellular and tissue interactions.Philos Trans R Soc Lond B Biol Sci. 1998 Jun 29;353(1370):915-23. doi: 10.1098/rstb.1998.0256. Philos Trans R Soc Lond B Biol Sci. 1998. PMID: 9684289 Free PMC article. Review.
-
Application of the Apc(Min/+) mouse model for studying inflammation-associated intestinal tumor.Biomed Pharmacother. 2015 Apr;71:216-21. doi: 10.1016/j.biopha.2015.02.023. Epub 2015 Feb 24. Biomed Pharmacother. 2015. PMID: 25960239 Review.
Cited by
-
Autophagy in colorectal cancer: An important switch from physiology to pathology.World J Gastrointest Oncol. 2015 Nov 15;7(11):271-84. doi: 10.4251/wjgo.v7.i11.271. World J Gastrointest Oncol. 2015. PMID: 26600927 Free PMC article. Review.
-
Natural Compounds _targeting the Autophagy Pathway in the Treatment of Colorectal Cancer.Int J Mol Sci. 2023 Apr 15;24(8):7310. doi: 10.3390/ijms24087310. Int J Mol Sci. 2023. PMID: 37108476 Free PMC article. Review.
-
Exploring heterogeneity: a dive into preclinical models of cancer cachexia.Am J Physiol Cell Physiol. 2024 Aug 1;327(2):C310-C328. doi: 10.1152/ajpcell.00317.2024. Epub 2024 Jun 10. Am J Physiol Cell Physiol. 2024. PMID: 38853648 Review.
-
Intestinal Autophagy and Its Pharmacological Control in Inflammatory Bowel Disease.Front Immunol. 2017 Jan 9;7:695. doi: 10.3389/fimmu.2016.00695. eCollection 2016. Front Immunol. 2017. PMID: 28119697 Free PMC article. Review.
-
The application of ApcMin/+ mouse model in colorectal tumor researches.J Cancer Res Clin Oncol. 2019 May;145(5):1111-1122. doi: 10.1007/s00432-019-02883-6. Epub 2019 Mar 18. J Cancer Res Clin Oncol. 2019. PMID: 30887153 Review.
References
-
- Jacoby RF, Seibert K, Cole CE, Kelloff G, Lubet RA. Cyclooxygenase-2 inhibitor celecoxib is a potent preventive and therapeutic agent in the Min mouse model of adenomatous polyposis. Cancer Res 2000; 60:5040-4; PMID:11016626 - PubMed
-
- Chau I, Cunningham D. Adjuvant therapy in colon cancer–what, when and how? Ann Oncol 2006; 17:1347-59; PMID:16524974; http://dx.doi.org/10.1093/annonc/mdl029 - DOI - PubMed
-
- Huerta S, Goulet EJ, Livingston EH. Colon cancer and apoptosis. Am J Surg 2006; 191:517-26; PMID:16531147; http://dx.doi.org/10.1016/j.amjsurg.2005.11.009 - DOI - PubMed
-
- Fodde R, Smits R, Clevers H. APC, signal transduction and genetic instability in colorectal cancer. Nat Rev Cancer 2001; 1:55-67; PMID:11900252; http://dx.doi.org/10.1038/35094067 - DOI - PubMed
-
- Ishikawa TO, Tamai Y, Li Q, Oshima M, Taketo MM. Requirement for tumor suppressor Apc in the morphogenesis of anterior and ventral mouse embryo. Dev Biol 2003; 253:230-46; PMID:12645927; http://dx.doi.org/10.1016/S0012-1606(02)00020-9 - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous
