Effects of Fatty Acid Amide Hydrolase (FAAH) Inhibitors in Non-Human Primate Models of Nicotine Reward and Relapse
- PMID: 25754762
- PMCID: PMC4613608
- DOI: 10.1038/npp.2015.62
Effects of Fatty Acid Amide Hydrolase (FAAH) Inhibitors in Non-Human Primate Models of Nicotine Reward and Relapse
Abstract
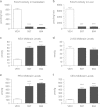
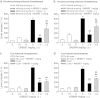
Inhibition of the enzyme fatty acid amide hydrolase (FAAH) counteracts reward-related effects of nicotine in rats, but it has not been tested for this purpose in non-human primates. Therefore, we studied the effects of the first- and second-generation O-arylcarbamate-based FAAH inhibitors, URB597 (cyclohexyl carbamic acid 3'-carbamoyl-3-yl ester) and URB694 (6-hydroxy-[1,1'-biphenyl]-3-yl-cyclohexylcarbamate), in squirrel monkeys. Both FAAH inhibitors: (1) blocked FAAH activity in brain and liver, increasing levels of endogenous ligands for cannabinoid and α-type peroxisome proliferator-activated (PPAR-α) receptors; (2) shifted nicotine self-administration dose-response functions in a manner consistent with reduced nicotine reward; (3) blocked reinstatement of nicotine seeking induced by reexposure to either nicotine priming or nicotine-associated cues; and (4) had no effect on cocaine or food self-administration. The effects of FAAH inhibition on nicotine self-administration and nicotine priming-induced reinstatement were reversed by the PPAR-α antagonist, MK886. Unlike URB597, which was not self-administered by monkeys in an earlier study, URB694 was self-administered at a moderate rate. URB694 self-administration was blocked by pretreatment with an antagonist for either PPAR-α (MK886) or cannabinoid CB1 receptors (rimonabant). In additional experiments in rats, URB694 was devoid of THC-like or nicotine-like interoceptive effects under drug-discrimination procedures, and neither of the FAAH inhibitors induced dopamine release in the nucleus accumbens shell--consistent with their lack of robust reinforcing effects in monkeys. Overall, both URB597 and URB694 show promise for the initialization and maintenance of smoking cessation because of their ability to block the rewarding effects of nicotine and prevent nicotine priming-induced and cue-induced reinstatement.
Figures



Similar articles
-
Blockade of nicotine reward and reinstatement by activation of alpha-type peroxisome proliferator-activated receptors.Biol Psychiatry. 2011 Apr 1;69(7):633-41. doi: 10.1016/j.biopsych.2010.07.009. Biol Psychiatry. 2011. PMID: 20801430 Free PMC article.
-
Attenuation of cue-induced reinstatement of nicotine seeking by URB597 through cannabinoid CB1 receptor in rats.Psychopharmacology (Berl). 2016 May;233(10):1823-8. doi: 10.1007/s00213-016-4232-y. Epub 2016 Feb 11. Psychopharmacology (Berl). 2016. PMID: 26864774
-
Self-administration of the anandamide transport inhibitor AM404 by squirrel monkeys.Psychopharmacology (Berl). 2016 May;233(10):1867-77. doi: 10.1007/s00213-016-4211-3. Epub 2016 Jan 23. Psychopharmacology (Berl). 2016. PMID: 26803499 Free PMC article.
-
The role of fatty acid amide hydrolase inhibition in nicotine reward and dependence.Life Sci. 2013 Mar 19;92(8-9):458-62. doi: 10.1016/j.lfs.2012.05.015. Epub 2012 Jun 12. Life Sci. 2013. PMID: 22705310 Free PMC article. Review.
-
6-Hydroxy-[1,1'-biphenyl]-3-yl-cyclohexyl-[11C-carbonyl]carbamate.2012 Oct 24 [updated 2013 Mar 21]. In: Molecular Imaging and Contrast Agent Database (MICAD) [Internet]. Bethesda (MD): National Center for Biotechnology Information (US); 2004–2013. 2012 Oct 24 [updated 2013 Mar 21]. In: Molecular Imaging and Contrast Agent Database (MICAD) [Internet]. Bethesda (MD): National Center for Biotechnology Information (US); 2004–2013. PMID: 23409303 Free Books & Documents. Review.
Cited by
-
Reinforcing effectiveness of nicotine in nonhuman primates: effects of nicotine dose and history of nicotine self-administration.Psychopharmacology (Berl). 2016 Jul;233(13):2451-8. doi: 10.1007/s00213-016-4293-y. Epub 2016 Apr 14. Psychopharmacology (Berl). 2016. PMID: 27076210 Free PMC article.
-
Exercise as an adjunctive treatment for cannabis use disorder.Am J Drug Alcohol Abuse. 2016 Sep;42(5):481-489. doi: 10.1080/00952990.2016.1185434. Epub 2016 Jun 17. Am J Drug Alcohol Abuse. 2016. PMID: 27314543 Free PMC article. Review.
-
The Role of Physical Exercise in Opioid Substitution Therapy: Mechanisms of Sequential Effects.Int J Mol Sci. 2023 Mar 1;24(5):4763. doi: 10.3390/ijms24054763. Int J Mol Sci. 2023. PMID: 36902190 Free PMC article. Review.
-
Effects of fatty acid amide hydrolase inhibitor URB597 in a rat model of trauma-induced long-term anxiety.Psychopharmacology (Berl). 2018 Nov;235(11):3211-3221. doi: 10.1007/s00213-018-5020-7. Epub 2018 Sep 24. Psychopharmacology (Berl). 2018. PMID: 30251159
-
Potentiation of endocannabinoids and other lipid amides prevents hyperalgesia and inflammation in a pre-clinical model of migraine.J Headache Pain. 2022 Jul 7;23(1):79. doi: 10.1186/s10194-022-01449-1. J Headache Pain. 2022. PMID: 35799128 Free PMC article.
References
-
- Astarita G, Ahmed F, Piomelli D (2008). Identification of biosynthetic precursors for the endocannabinoid anandamide in the rat brain. J Lipid Res 49: 48–57. - PubMed
-
- Cravatt BF, Giang DK, Mayfield SP, Boger DL, Lerner RA, Gilula NB (1996). Molecular characterization of an enzyme that degrades neuromodulatory fatty-acid amides. Nature 384: 83–87. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

