Translational arrest due to cytoplasmic redox stress delays adaptation to growth on methanol and heterologous protein expression in a typical fed-batch culture of Pichia pastoris
- PMID: 25785713
- PMCID: PMC4364781
- DOI: 10.1371/journal.pone.0119637
Translational arrest due to cytoplasmic redox stress delays adaptation to growth on methanol and heterologous protein expression in a typical fed-batch culture of Pichia pastoris
Abstract
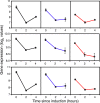
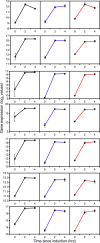
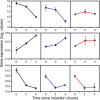
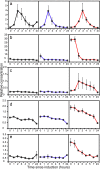
Results: We have followed a typical fed-batch induction regime for heterologous protein production under the control of the AOX1 promoter using both microarray and metabolomic analysis. The genetic constructs involved 1 and 3 copies of the TRY1 gene, encoding human trypsinogen. In small-scale laboratory cultures, expression of the 3 copy-number construct induced the unfolded protein response (UPR) sufficiently that titres of extracellular trypsinogen were lower in the 3-copy construct than with the 1-copy construct. In the fed-batch-culture, a similar pattern was observed, with higher expression from the 1-copy construct, but in this case there was no significant induction of UPR with the 3-copy strain. Analysis of the microarray and metabolomic information indicates that the 3-copy strain was undergoing cytoplasmic redox stress at the point of induction with methanol. In this Crabtree-negative yeast, this redox stress appeared to delay the adaptation to growth on methanol and supressed heterologous protein production, probably due to a block in translation.
Conclusion: Although redox imbalance as a result of artificially imposed hypoxia has previously been described, this is the first time that it has been characterised as a result of a transient metabolic imbalance and shown to involve a stress response which can lead to translational arrest. Without detailed analysis of the underlying processes it could easily have been mis-interpreted as secretion stress, transmitted through the UPR.
Conflict of interest statement
Figures
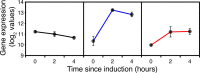
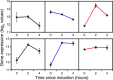
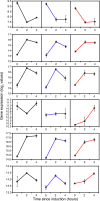
Similar articles
-
Decrease of UPR- and ERAD-related proteins in Pichia pastoris during methanol-induced secretory insulin precursor production in controlled fed-batch cultures.Microb Cell Fact. 2014 Feb 13;13(1):23. doi: 10.1186/1475-2859-13-23. Microb Cell Fact. 2014. PMID: 24521445 Free PMC article.
-
Fate of the UPR marker protein Kar2/Bip and autophagic processes in fed-batch cultures of secretory insulin precursor producing Pichia pastoris.Microb Cell Fact. 2018 Aug 9;17(1):123. doi: 10.1186/s12934-018-0970-3. Microb Cell Fact. 2018. PMID: 30092809 Free PMC article.
-
Increased dosage of AOX1 promoter-regulated expression cassettes leads to transcription attenuation of the methanol metabolism in Pichia pastoris.Sci Rep. 2017 Mar 15;7:44302. doi: 10.1038/srep44302. Sci Rep. 2017. PMID: 28295011 Free PMC article.
-
Integrating metabolic modeling and population heterogeneity analysis into optimizing recombinant protein production by Komagataella (Pichia) pastoris.Appl Microbiol Biotechnol. 2018 Jan;102(1):63-80. doi: 10.1007/s00253-017-8612-y. Epub 2017 Nov 14. Appl Microbiol Biotechnol. 2018. PMID: 29138907 Review.
-
Engineering strategies for enhanced production of protein and bio-products in Pichia pastoris: A review.Biotechnol Adv. 2018 Jan-Feb;36(1):182-195. doi: 10.1016/j.biotechadv.2017.11.002. Epub 2017 Nov 10. Biotechnol Adv. 2018. PMID: 29129652 Review.
Cited by
-
Identification of novel factors enhancing recombinant protein production in multi-copy Komagataella phaffii based on transcriptomic analysis of overexpression effects.Sci Rep. 2017 Nov 24;7(1):16249. doi: 10.1038/s41598-017-16577-x. Sci Rep. 2017. PMID: 29176680 Free PMC article.
-
Rapid screening of cellular stress responses in recombinant Pichia pastoris strains using metabolite profiling.J Ind Microbiol Biotechnol. 2017 Mar;44(3):413-417. doi: 10.1007/s10295-017-1904-5. Epub 2017 Feb 3. J Ind Microbiol Biotechnol. 2017. PMID: 28160205 Free PMC article.
-
Single-Cell Approach to Monitor the Unfolded Protein Response During Biotechnological Processes With Pichia pastoris.Front Microbiol. 2019 Feb 27;10:335. doi: 10.3389/fmicb.2019.00335. eCollection 2019. Front Microbiol. 2019. PMID: 30873140 Free PMC article.
-
Cofactor engineering improved CALB production in Pichia pastoris through heterologous expression of NADH oxidase and adenylate kinase.PLoS One. 2017 Jul 17;12(7):e0181370. doi: 10.1371/journal.pone.0181370. eCollection 2017. PLoS One. 2017. PMID: 28715469 Free PMC article.
-
Expressing anti-HIV VRC01 antibody using the murine IgG1 secretion signal in Pichia pastoris.AMB Express. 2017 Dec;7(1):70. doi: 10.1186/s13568-017-0372-7. Epub 2017 Mar 24. AMB Express. 2017. PMID: 28342171 Free PMC article.
References
-
- Macauley-Patrick S, Fazenda ML, McNeil B, Harvey LM. Heterologous protein production using the Pichia pastoris expression system. Yeast. 2005. Mar;22(4):249–70. - PubMed
-
- Sreekrishna K, Flickinger MC. Pichia, Optimization of Protein Expression Encyclopedia of Industrial Biotechnology: John Wiley & Sons, Inc.; 2009.
-
- Cereghino JL, Cregg JM. Heterologous protein expression in the methylotrophic yeast Pichia pastoris. FEMS Microbiology Reviews 2000. 24:45–66. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

