MG53-mediated cell membrane repair protects against acute kidney injury
- PMID: 25787762
- PMCID: PMC4524523
- DOI: 10.1126/scitranslmed.3010755
MG53-mediated cell membrane repair protects against acute kidney injury
Abstract
Injury to the renal proximal tubular epithelium (PTE) represents the underlying consequence of acute kidney injury (AKI) after exposure to various stressors, including nephrotoxins and ischemia/reperfusion (I/R). Although the kidney has the ability to repair itself after mild injury, insufficient repair of PTE cells may trigger inflammatory and fibrotic responses, leading to chronic renal failure. We report that MG53, a member of the TRIM family of proteins, participates in repair of injured PTE cells and protects against the development of AKI. We show that MG53 translocates to acute injury sites on PTE cells and forms a repair patch. Ablation of MG53 leads to defective membrane repair. MG53-deficient mice develop pronounced tubulointerstitial injury and increased susceptibility to I/R-induced AKI compared to wild-type mice. Recombinant human MG53 (rhMG53) protein can _target injury sites on PTE cells to facilitate repair after I/R injury or nephrotoxin exposure. Moreover, in animal studies, intravenous delivery of rhMG53 ameliorates cisplatin-induced AKI without affecting the tumor suppressor efficacy of cisplatin. These findings identify MG53 as a vital component of reno-protection, and _targeting MG53-mediated repair of PTE cells represents a potential approach to prevention and treatment of AKI.
Copyright © 2015, American Association for the Advancement of Science.
Figures

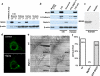
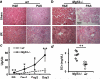
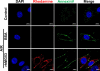
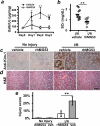
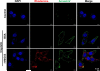
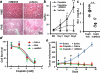

Comment in
-
Acute kidney injury: Mitsugumin 53 mediates repair of the damaged proximal tubular epithelium.Nat Rev Nephrol. 2015 May;11(5):253. doi: 10.1038/nrneph.2015.50. Epub 2015 Apr 7. Nat Rev Nephrol. 2015. PMID: 25848877 No abstract available.
Similar articles
-
MG53 protects against contrast-induced acute kidney injury by reducing cell membrane damage and apoptosis.Acta Pharmacol Sin. 2020 Nov;41(11):1457-1464. doi: 10.1038/s41401-020-0420-8. Epub 2020 May 18. Acta Pharmacol Sin. 2020. PMID: 32424239 Free PMC article.
-
Treatment of acute lung injury by _targeting MG53-mediated cell membrane repair.Nat Commun. 2014 Jul 18;5:4387. doi: 10.1038/ncomms5387. Nat Commun. 2014. PMID: 25034454 Free PMC article.
-
MG53 permeates through blood-brain barrier to protect ischemic brain injury.Onco_target. 2016 Apr 19;7(16):22474-85. doi: 10.18632/onco_target.7965. Onco_target. 2016. PMID: 26967557 Free PMC article.
-
MG53: A potential therapeutic _target for kidney disease.Pharmacol Res Perspect. 2023 Feb;11(1):e01049. doi: 10.1002/prp2.1049. Pharmacol Res Perspect. 2023. PMID: 36583464 Free PMC article. Review.
-
MG53: Biological Function and Potential as a Therapeutic _target.Mol Pharmacol. 2017 Sep;92(3):211-218. doi: 10.1124/mol.117.108241. Epub 2017 Apr 21. Mol Pharmacol. 2017. PMID: 28432201 Review.
Cited by
-
Acute kidney injury: Mitsugumin 53 mediates repair of the damaged proximal tubular epithelium.Nat Rev Nephrol. 2015 May;11(5):253. doi: 10.1038/nrneph.2015.50. Epub 2015 Apr 7. Nat Rev Nephrol. 2015. PMID: 25848877 No abstract available.
-
An actin-dependent annexin complex mediates plasma membrane repair in muscle.J Cell Biol. 2016 Jun 20;213(6):705-18. doi: 10.1083/jcb.201512022. Epub 2016 Jun 13. J Cell Biol. 2016. PMID: 27298325 Free PMC article.
-
Structural basis for TRIM72 oligomerization during membrane damage repair.Nat Commun. 2023 Mar 21;14(1):1555. doi: 10.1038/s41467-023-37198-1. Nat Commun. 2023. PMID: 36944613 Free PMC article.
-
Sustained delivery of rhMG53 promotes diabetic wound healing and hair follicle development.Bioact Mater. 2022 Mar 16;18:104-115. doi: 10.1016/j.bioactmat.2022.03.017. eCollection 2022 Dec. Bioact Mater. 2022. PMID: 35387169 Free PMC article.
-
MG53 Mitigates Nitrogen Mustard-Induced Skin Injury.Cells. 2023 Jul 23;12(14):1915. doi: 10.3390/cells12141915. Cells. 2023. PMID: 37508578 Free PMC article.
References
-
- Christensen EI, Birn H, Storm T, Weyer K, Nielsen R. Endocytic receptors in the renal proximal tubule. Physiology. 2012;27:223–236. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- HL084583/HL/NHLBI NIH HHS/United States
- AG028614/AG/NIA NIH HHS/United States
- R01 HL114383/HL/NHLBI NIH HHS/United States
- HL069000/HL/NHLBI NIH HHS/United States
- R01 HL083422/HL/NHLBI NIH HHS/United States
- HL083422/HL/NHLBI NIH HHS/United States
- U54 CA163111/CA/NCI NIH HHS/United States
- R01 HL084583/HL/NHLBI NIH HHS/United States
- R01 HL069000/HL/NHLBI NIH HHS/United States
- AR061385/AR/NIAMS NIH HHS/United States
- R01 AG028614/AG/NIA NIH HHS/United States
- R01 AR061385/AR/NIAMS NIH HHS/United States
- U01 DK096927/DK/NIDDK NIH HHS/United States
- HL114383/HL/NHLBI NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

