Comparison between the cultures of human induced pluripotent stem cells (hiPSCs) on feeder-and serum-free system (Matrigel matrix), MEF and HDF feeder cell lines
- PMID: 25820945
- PMCID: PMC4580685
- DOI: 10.1007/s12079-015-0289-3
Comparison between the cultures of human induced pluripotent stem cells (hiPSCs) on feeder-and serum-free system (Matrigel matrix), MEF and HDF feeder cell lines
Abstract
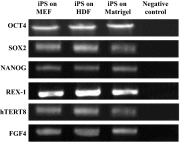
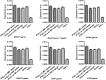
Human induced pluripotent stem cells (hiPSCs) are a type of pluripotent stem cells artificially derived from an adult somatic cell (typically human fibroblast) by forced expression of specific genes. In recent years, different feeders like inactivated mouse embryonic fibroblasts (MEFs), human dermal fibroblasts (HDFs), and feeder free system have commonly been used for supporting the culture of stem cells in undifferentiated state. In the present work, the culture of hiPSCs and their characterizations on BD Matrigel (feeder-and serum-free system), MEF and HDF feeders using cell culture methods and molecular techniques were evaluated and compared. The isolated HDFs from foreskin samples were reprogrammed to hiPSCs using gene delivery system. Then, the pluripotency ability of hiPSCs cultured on each layer was determined by teratoma formation and immunohistochemical staining. After EBs generation the expression level of three germ layers genes were evaluated by Q-real-time PCR. Also, the cytogenetic stability of hiPSCs cultured on each condition was analyzed by karyotyping and comet assay. Then, the presence of pluripotency antigens were confirmed by Immunocytochemistry (ICC) test and alkaline phosphatase staining. This study were showed culturing of hiPSCs on BD Matrigel, MEF and HDF feeders had normal morphology and could maintain in undifferentiated state for prolonged expansion. The hiPSCs cultured in each system had normal karyotype without any chromosomal abnormalities and the DNA lesions were not observed by comet assay. Moreover, up-regulation in three germ layers genes in cultured hiPSCs on each layer (same to ESCs) compare to normal HDFs were observed (p < 0.05). The findings of the present work were showed in stem cells culturing especially hiPSCs both MEF and HDF feeders as well as feeder free system like Matrigel are proper despite benefits and disadvantages. Although, MEFs is suitable for supporting of stem cell culturing but it can animal pathogens transferring and inducing immune response. Furthermore, HDFs have homologous source with hiPSCs and can be used as feeder instead of MEF but in therapeutic approaches the cells contamination is a problem. So, this study were suggested feeder free culturing of hiPSCs on Matrigel in supplemented media (without using MEF conditioned medium) resolves these problems and could prepare easy applications of hiPSCs in therapeutic approaches of regenerative medicine such as stem-cell therapy and somatic cell nuclear in further researches.
Keywords: BD Matrigel matrix ICC; Comet assay; HDF; IHC; Karyotyping; MEF; hiPSCs.
Figures






Similar articles
-
Preparation of mouse embryonic fibroblast cells suitable for culturing human embryonic and induced pluripotent stem cells.J Vis Exp. 2012 Jun 21;(64):3854. doi: 10.3791/3854. J Vis Exp. 2012. PMID: 22760161 Free PMC article.
-
Gingival Fibroblasts as Autologous Feeders for Induced Pluripotent Stem Cells.J Dent Res. 2016 Jan;95(1):110-8. doi: 10.1177/0022034515611602. Epub 2015 Oct 14. J Dent Res. 2016. PMID: 26467419
-
Human iPS cell-derived fibroblast-like cells as feeder layers for iPS cell derivation and expansion.J Biosci Bioeng. 2015 Aug;120(2):210-7. doi: 10.1016/j.jbiosc.2014.12.009. Epub 2015 Jan 24. J Biosci Bioeng. 2015. PMID: 25622768
-
Conditioning pluripotent stem cell media with mouse embryonic fibroblasts (MEF-CM).2012 Jun 10. In: StemBook [Internet]. Cambridge (MA): Harvard Stem Cell Institute; 2008–. 2012 Jun 10. In: StemBook [Internet]. Cambridge (MA): Harvard Stem Cell Institute; 2008–. PMID: 23658989 Free Books & Documents. Review.
-
Xeno-free culture and proliferation of hPSCs on 2D biomaterials.Prog Mol Biol Transl Sci. 2023;199:63-107. doi: 10.1016/bs.pmbts.2023.02.008. Epub 2023 Mar 14. Prog Mol Biol Transl Sci. 2023. PMID: 37678982 Review.
Cited by
-
Increased levels of miR-124 in human dental pulp stem cells alter the expression of neural markers.J Otol. 2019 Dec;14(4):121-127. doi: 10.1016/j.joto.2019.04.001. Epub 2019 Apr 16. J Otol. 2019. PMID: 32742271 Free PMC article.
-
Robust protocol for feeder-free adaptation of cryopreserved human pluripotent stem cells.In Vitro Cell Dev Biol Anim. 2019 Dec;55(10):777-783. doi: 10.1007/s11626-019-00413-9. Epub 2019 Oct 29. In Vitro Cell Dev Biol Anim. 2019. PMID: 31664691
-
Growing Glia: Cultivating Human Stem Cell Models of Gliogenesis in Health and Disease.Front Cell Dev Biol. 2021 Mar 25;9:649538. doi: 10.3389/fcell.2021.649538. eCollection 2021. Front Cell Dev Biol. 2021. PMID: 33842475 Free PMC article. Review.
-
Protein biomarkers of neural system.J Otol. 2019 Sep;14(3):77-88. doi: 10.1016/j.joto.2019.03.001. Epub 2019 Mar 23. J Otol. 2019. PMID: 31467504 Free PMC article. Review.
-
Droplet Microarray Based Screening Identifies Proteins for Maintaining Pluripotency of hiPSCs.Adv Healthc Mater. 2022 Sep;11(18):e2200718. doi: 10.1002/adhm.202200718. Epub 2022 Jul 20. Adv Healthc Mater. 2022. PMID: 35799451 Free PMC article.
References
-
- Aguilar-Gallardo C, Poo M, Gomez E, Galan A, Sanchez E, Marques-Mari A, Ruiz V, Medrano J, Riboldi M, Valbuena D, Simon C. Derivation, characterization, differentiation, and registration of seven human embryonic stem cell lines (VAL-3, −4, −5, −6 M, −7, −8, and −9) on human feeder. In Vitro Cell Dev Biol Anim. 2010;46(3–4):317–326. doi: 10.1007/s11626-010-9285-3. - DOI - PubMed
-
- Bisson F, Rochefort É, Lavoie A, Larouche D, Zaniolo K, Simard-Bisson C, Damour O, Auger FA, Guérin SL, Germain L. Irradiated human dermal fibroblasts are as efficient as mouse fibroblasts as a feeder layer to improve human epidermal cell culture lifespan. Int J Mol Sci. 2013;14(3):4684–4704. doi: 10.3390/ijms14034684. - DOI - PMC - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources

