Impaired NK Cell Responses to Pertussis and H1N1 Influenza Vaccine Antigens in Human Cytomegalovirus-Infected Individuals
- PMID: 25855356
- PMCID: PMC4416741
- DOI: 10.4049/jimmunol.1403080
Impaired NK Cell Responses to Pertussis and H1N1 Influenza Vaccine Antigens in Human Cytomegalovirus-Infected Individuals
Abstract
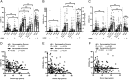
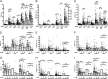
NK cells contribute to postvaccination immune responses after activation by IL-2 from Ag-specific memory T cells or by cross-linking of the low-affinity IgG receptor, CD16, by Ag-Ab immune complexes. Sensitivity of NK cells to these signals from the adaptive immune system is heterogeneous and influenced by their stage of differentiation. CD56(dim)CD57(+) NK cells are less responsive to IL-2 and produce less IFN-γ in response to T cell-mediated activation than do CD56(bright) or CD56(dim)CD57(-) NK cells. Conversely, NK cell cytotoxicity, as measured by degranulation, is maintained across the CD56(dim) subsets. Human CMV (HCMV), a highly prevalent herpes virus causing lifelong, usually latent, infections, drives the expansion of the CD56(dim)CD57(+)NKG2C(+) NK cell population, skewing the NK cell repertoire in favor of cytotoxic responses at the expense of cytokine-driven responses. We hypothesized, therefore, that HCMV seropositivity would be associated with altered NK cell responses to vaccine Ags. In a cross-sectional study of 152 U.K. adults, with HCMV seroprevalence rate of 36%, we find that HCMV seropositivity is associated with lower NK cell IFN-γ production and degranulation after in vitro restimulation with pertussis or H1N1 influenza vaccine Ags. Higher expression of CD57/NKG2C and lower expression of IL-18Rα on NK cells from HCMV seropositive subjects do not fully explain these impaired responses, which are likely the result of multiple receptor-ligand interactions. This study demonstrates for the first time, to our knowledge, that HCMV serostatus influences NK cell contributions to adaptive immunity and raises important questions regarding the impact of HCMV infection on vaccine efficacy.
Copyright © 2015 The Authors.
Figures




Similar articles
-
Enhancement of cytokine-driven NK cell IFN-γ production after vaccination of HCMV infected Africans.Eur J Immunol. 2017 Jun;47(6):1040-1050. doi: 10.1002/eji.201746974. Epub 2017 Apr 24. Eur J Immunol. 2017. PMID: 28383105 Free PMC article.
-
Late Development of FcεRγneg Adaptive Natural Killer Cells Upon Human Cytomegalovirus Reactivation in Umbilical Cord Blood Transplantation Recipients.Front Immunol. 2018 May 15;9:1050. doi: 10.3389/fimmu.2018.01050. eCollection 2018. Front Immunol. 2018. PMID: 29868012 Free PMC article.
-
Influenza Vaccination Generates Cytokine-Induced Memory-like NK Cells: Impact of Human Cytomegalovirus Infection.J Immunol. 2016 Jul 1;197(1):313-25. doi: 10.4049/jimmunol.1502049. Epub 2016 May 27. J Immunol. 2016. PMID: 27233958 Free PMC article.
-
CMV induces rapid NK cell maturation in HSCT recipients.Immunol Lett. 2013 Sep-Oct;155(1-2):11-3. doi: 10.1016/j.imlet.2013.09.020. Epub 2013 Sep 26. Immunol Lett. 2013. PMID: 24076315 Review.
-
The CD94/NKG2C+ NK-cell subset on the edge of innate and adaptive immunity to human cytomegalovirus infection.Semin Immunol. 2014 Apr;26(2):145-51. doi: 10.1016/j.smim.2014.03.002. Epub 2014 Mar 22. Semin Immunol. 2014. PMID: 24666761 Review.
Cited by
-
Human Immunodeficiency Virus Exposure but Not Early Cytomegalovirus Infection Is Associated With Increased Hospitalization and Decreased Memory T-Cell Responses to Tetanus Vaccine.J Infect Dis. 2020 Mar 16;221(7):1167-1175. doi: 10.1093/infdis/jiz590. J Infect Dis. 2020. PMID: 31711179 Free PMC article.
-
IL-15 Promotes Polyfunctional NK Cell Responses to Influenza by Boosting IL-12 Production.J Immunol. 2018 Apr 15;200(8):2738-2747. doi: 10.4049/jimmunol.1701614. Epub 2018 Feb 28. J Immunol. 2018. PMID: 29491009 Free PMC article.
-
Assessment of the Interferon-Lambda-3 Polymorphism in the Antibody Response to COVID-19 in Older Adults Seropositive for CMV.Vaccines (Basel). 2023 Feb 18;11(2):480. doi: 10.3390/vaccines11020480. Vaccines (Basel). 2023. PMID: 36851357 Free PMC article.
-
Enhancement of cytokine-driven NK cell IFN-γ production after vaccination of HCMV infected Africans.Eur J Immunol. 2017 Jun;47(6):1040-1050. doi: 10.1002/eji.201746974. Epub 2017 Apr 24. Eur J Immunol. 2017. PMID: 28383105 Free PMC article.
-
Cytomegalovirus Infection May Contribute to the Reduced Immune Function, Growth, Development, and Health of HIV-Exposed, Uninfected African Children.Front Immunol. 2016 Jun 30;7:257. doi: 10.3389/fimmu.2016.00257. eCollection 2016. Front Immunol. 2016. PMID: 27446087 Free PMC article. Review.
References
-
- Horowitz A., Behrens R. H., Okell L., Fooks A. R., Riley E. M. 2010. NK cells as effectors of acquired immune responses: effector CD4+ T cell-dependent activation of NK cells following vaccination. J. Immunol. 185: 2808–2818. - PubMed
-
- Horowitz A., Hafalla J. C., King E., Lusingu J., Dekker D., Leach A., Moris P., Cohen J., Vekemans J., Villafana T., et al. 2012. Antigen-specific IL-2 secretion correlates with NK cell responses after immunization of Tanzanian children with the RTS,S/AS01 malaria vaccine. J. Immunol. 188: 5054–5062. - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

