Crystal structure of ethyl 6-methyl-2-sulfanyl-idene-4-(thio-phen-2-yl)-1,2,3,4-tetra-hydro-pyrimidine-5-carboxyl-ate
- PMID: 25878882
- PMCID: PMC4384618
- DOI: 10.1107/S2056989014027741
Crystal structure of ethyl 6-methyl-2-sulfanyl-idene-4-(thio-phen-2-yl)-1,2,3,4-tetra-hydro-pyrimidine-5-carboxyl-ate
Abstract
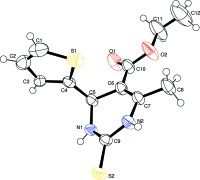
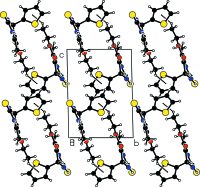
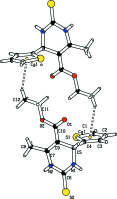
In the title compound, C12H14N2O2S2, the di-hydro-pyrimidine ring adopts a sofa conformation, with the C atom bearing the thienyl ring lying above the plane of the five remaining approximately coplanar (r.m.s. deviation = 0.0405 Å) atoms of the ring. The dihedral angle between the five near coplanar atoms of the ring and the thienyl ring is 89.78 (11)°. In the crystal, mol-ecules are linked into a supra-molecular chain along [100] via N-H⋯O(carbon-yl) hydrogen bonds. Inversion-related chains are linked into double chains via N-H⋯S(thione) hydrogen bonds. The three-dimensional architecture also features meth-yl-thienyl C-H⋯π inter-actions.
Keywords: C—H⋯π interactions; conformation; crystal structure; hydrogen bonding; pyrimidine.
Figures
Similar articles
-
Crystal structure of ethyl 6-(2-fluoro-phen-yl)-4-hy-droxy-2-sulfanyl-idene-4-tri-fluoro-meth-yl-1,3-diazinane-5-carboxyl-ate.Acta Crystallogr E Crystallogr Commun. 2015 Apr 2;71(Pt 5):o268-9. doi: 10.1107/S2056989015005836. eCollection 2015 May 1. Acta Crystallogr E Crystallogr Commun. 2015. PMID: 25995898 Free PMC article.
-
Ethyl 6-methyl-2-sulfanyl-idene-4-[4-(trifluoro-meth-yl)phen-yl]-1,2,3,4-tetra-hydro-pyrimidine-5-carboxyl-ate.Acta Crystallogr Sect E Struct Rep Online. 2011 Jul 1;67(Pt 7):o1559-60. doi: 10.1107/S1600536811019441. Epub 2011 Jun 4. Acta Crystallogr Sect E Struct Rep Online. 2011. PMID: 21836973 Free PMC article.
-
Crystal structure of ethyl 6-chloro-methyl-2-oxo-4-(2,3,4-tri-meth-oxy-phen-yl)-1,2,3,4-tetra-hydro-pyrimidine-5-carboxyl-ate.Acta Crystallogr E Crystallogr Commun. 2015 Jun 20;71(Pt 7):821-3. doi: 10.1107/S2056989015011688. eCollection 2015 Jul 1. Acta Crystallogr E Crystallogr Commun. 2015. PMID: 26279876 Free PMC article.
-
Crystal structure of ethyl 6-methyl-2-oxo-4-(3,4,5-tri-meth-oxy-phen-yl)-1,2,3,4-tetra-hydro-pyrimidine-5-carboxyl-ate.Acta Crystallogr E Crystallogr Commun. 2015 Feb 25;71(Pt 3):o206-7. doi: 10.1107/S2056989015003576. eCollection 2015 Mar 1. Acta Crystallogr E Crystallogr Commun. 2015. PMID: 25844251 Free PMC article.
-
Methyl 4-(4-hy-droxy-phen-yl)-6-methyl-2-sulfanyl-idene-1,2,3,4-tetra-hydro-pyrimidine-5-carboxyl-ate.Acta Crystallogr Sect E Struct Rep Online. 2014 Feb 15;70(Pt 3):o306. doi: 10.1107/S1600536814002888. eCollection 2014 Mar 1. Acta Crystallogr Sect E Struct Rep Online. 2014. PMID: 24765008 Free PMC article.
References
-
- Altomare, A., Cascarano, G., Giacovazzo, C. & Guagliardi, A. (1993). J. Appl. Cryst. 26, 343–350.
-
- Bruker (2008). APEX2, SADABS, SAINT and XPREP. Bruker AXS Inc., Madison, Wisconsin, USA.
-
- Farrugia, L. J. (2012). J. Appl. Cryst. 45, 849–854.
-
- Macrae, C. F., Bruno, I. J., Chisholm, J. A., Edgington, P. R., McCabe, P., Pidcock, E., Rodriguez-Monge, L., Taylor, R., van de Streek, J. & Wood, P. A. (2008). J. Appl. Cryst. 41, 466–470.
-
- Patil, S., Jadhav, S. D. & Deshmukh, M. B. (2011). Arch. Appl. Sci. Res. 3, 203–208.
LinkOut - more resources
Full Text Sources
Research Materials
