The effectiveness of high dose zinc acetate lozenges on various common cold symptoms: a meta-analysis
- PMID: 25888289
- PMCID: PMC4359576
- DOI: 10.1186/s12875-015-0237-6
The effectiveness of high dose zinc acetate lozenges on various common cold symptoms: a meta-analysis
Abstract
Background: A previous meta-analysis found that high dose zinc acetate lozenges reduced the duration of common colds by 42%, whereas low zinc doses had no effect. Lozenges are dissolved in the pharyngeal region, thus there might be some difference in the effect of zinc lozenges on the duration of respiratory symptoms in the pharyngeal region compared with the nasal region. The objective of this study was to determine whether zinc acetate lozenges have different effects on the duration of common cold symptoms originating from different anatomical regions.
Methods: We analyzed three randomized trials on zinc acetate lozenges for the common cold administering zinc in doses of 80-92 mg/day. All three trials reported the effect of zinc on seven respiratory symptoms, and three systemic symptoms. We pooled the effects of zinc lozenges for each symptom and calculated point estimates and 95% confidence intervals (95% CI).
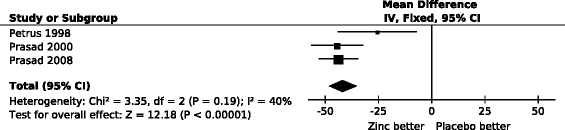
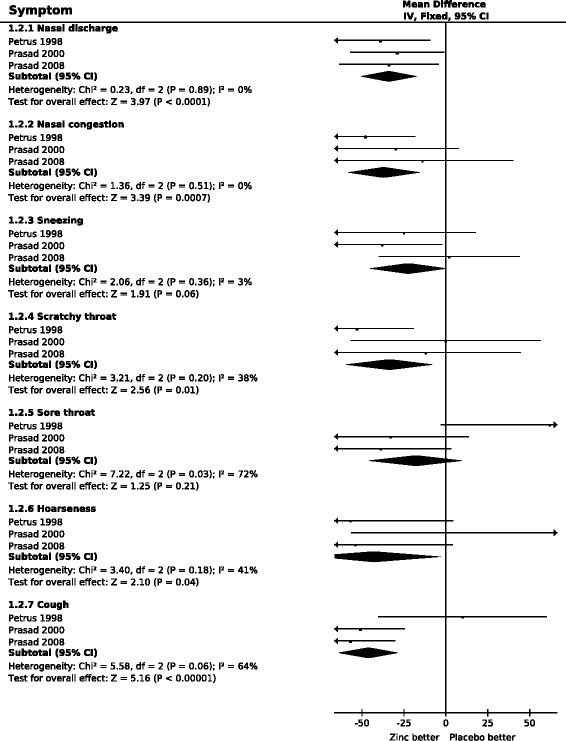
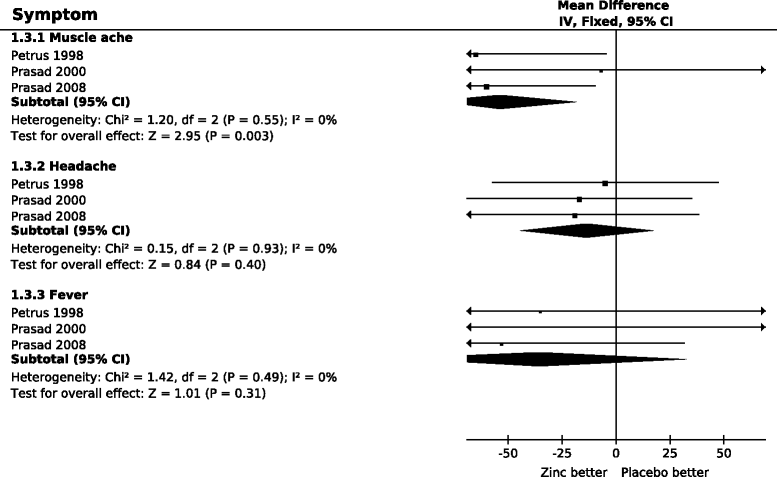
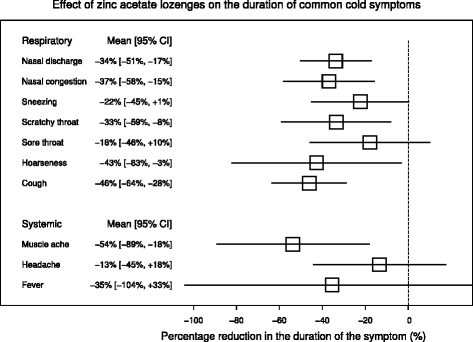
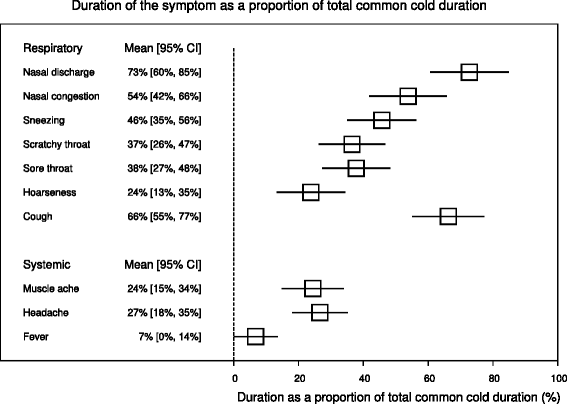
Results: Zinc acetate lozenges shortened the duration of nasal discharge by 34% (95% CI: 17% to 51%), nasal congestion by 37% (15% to 58%), sneezing by 22% (-1% to 45%), scratchy throat by 33% (8% to 59%), sore throat by 18% (-10% to 46%), hoarseness by 43% (3% to 83%), and cough by 46% (28% to 64%). Zinc lozenges shortened the duration of muscle ache by 54% (18% to 89%), but there was no difference in the duration of headache and fever.
Conclusions: The effect of zinc acetate lozenges on cold symptoms may be associated with the local availability of zinc from the lozenges, with the levels being highest in the pharyngeal region. However our findings indicate that the effects of zinc ions are not limited to the pharyngeal region. There is no indication that the effect of zinc lozenges on nasal symptoms is less than the effect on the symptoms of the pharyngeal region, which is more exposed to released zinc ions. Given that the adverse effects of zinc in the three trials were minor, zinc acetate lozenges releasing zinc ions at doses of about 80 mg/day may be a useful treatment for the common cold, started within 24 hours, for a time period of less than two weeks.
Figures
Similar articles
-
Zinc acetate lozenges for treating the common cold: an individual patient data meta-analysis.Br J Clin Pharmacol. 2016 Nov;82(5):1393-1398. doi: 10.1111/bcp.13057. Epub 2016 Jul 28. Br J Clin Pharmacol. 2016. PMID: 27378206 Free PMC article.
-
Duration of symptoms and plasma cytokine levels in patients with the common cold treated with zinc acetate. A randomized, double-blind, placebo-controlled trial.Ann Intern Med. 2000 Aug 15;133(4):245-52. doi: 10.7326/0003-4819-133-4-200008150-00006. Ann Intern Med. 2000. PMID: 10929163 Clinical Trial.
-
Zinc gluconate lozenges for treating the common cold. A randomized, double-blind, placebo-controlled study.Ann Intern Med. 1996 Jul 15;125(2):81-8. doi: 10.7326/0003-4819-125-2-199607150-00001. Ann Intern Med. 1996. PMID: 8678384 Clinical Trial.
-
The role of zinc lozenges in treatment of the common cold.Ann Pharmacother. 1998 Jan;32(1):63-9. doi: 10.1345/aph.17128. Ann Pharmacother. 1998. PMID: 9475824 Review.
-
Zinc lozenges as cure for the common cold--a review and hypothesis.Med Hypotheses. 2010 Mar;74(3):482-92. doi: 10.1016/j.mehy.2009.10.017. Epub 2009 Nov 10. Med Hypotheses. 2010. PMID: 19906491 Free PMC article. Review.
Cited by
-
Shortcomings in the Cochrane review on zinc for the common cold (2024).Front Med (Lausanne). 2024 Oct 16;11:1470004. doi: 10.3389/fmed.2024.1470004. eCollection 2024. Front Med (Lausanne). 2024. PMID: 39478818 Free PMC article. No abstract available.
-
Zinc for prevention and treatment of the common cold.Cochrane Database Syst Rev. 2024 May 9;5(5):CD014914. doi: 10.1002/14651858.CD014914.pub2. Cochrane Database Syst Rev. 2024. PMID: 38719213
-
Zinc acetate lozenges for treating the common cold: an individual patient data meta-analysis.Br J Clin Pharmacol. 2016 Nov;82(5):1393-1398. doi: 10.1111/bcp.13057. Epub 2016 Jul 28. Br J Clin Pharmacol. 2016. PMID: 27378206 Free PMC article.
-
Zinc Acetate Lozenges May Improve the Recovery Rate of Common Cold Patients: An Individual Patient Data Meta-Analysis.Open Forum Infect Dis. 2017 Apr 3;4(2):ofx059. doi: 10.1093/ofid/ofx059. eCollection 2017 Spring. Open Forum Infect Dis. 2017. PMID: 28480298 Free PMC article.
-
Zinc lozenges and the common cold: a meta-analysis comparing zinc acetate and zinc gluconate, and the role of zinc dosage.JRSM Open. 2017 May 2;8(5):2054270417694291. doi: 10.1177/2054270417694291. eCollection 2017 May. JRSM Open. 2017. PMID: 28515951 Free PMC article.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

