USP7 deubiquitinase promotes ubiquitin-dependent DNA damage signaling by stabilizing RNF168
- PMID: 25894431
- PMCID: PMC4613370
- DOI: 10.1080/15384101.2015.1007785
USP7 deubiquitinase promotes ubiquitin-dependent DNA damage signaling by stabilizing RNF168
Abstract
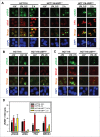
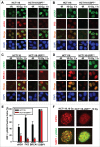
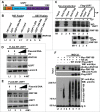
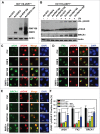
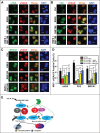
During DNA damage response (DDR), histone ubiquitination by RNF168 is a critical event, which orchestrates the recruitment of downstream DDR factors, e.g. BRCA1 and 53BP1. Here, we report USP7 deubiquitinase regulates the stability of RNF168. We showed that USP7 disruption impairs H2A and ultraviolet radiation (UVR)-induced γH2AX monoubiquitination, and decreases the levels of pBmi1, Bmi1, RNF168 and BRCA1. The effect of USP7 disruption was recapitulated by siRNA-mediated USP7 depletion. The USP7 disruption also compromises the formation of UVR-induced foci (UVRIF) and ionizing radiation-induced foci (IRIF) of monoubiquitinated H2A (uH2A) and polyubiquitinated H2AX/A, and subsequently affects UVRIF and IRIF of BRCA1 as well as the IRIF of 53BP1. USP7 was shown to physically bind RNF168 in vitro and in vivo. Overexpression of wild-type USP7, but not its interaction-defective mutant, prevents UVR-induced RNF168 degradation. The USP7 mutant is unable to cleave Ub-conjugates of RNF168 in vivo. Importantly, ectopic expression of RNF168, or both RNF8 and RNF168 together in USP7-disrupted cells, significantly rescue the formation of UVRIF and IRIF of polyubiquitinated H2A and BRCA1. Taken together, these findings reveal an important role of USP7 in regulating ubiquitin-dependent signaling via stabilization of RNF168.
Keywords: 53BP1; BRCA; DNA damage response; RNF168; USP7; deubiquitinating enzyme; histone modification.
Figures

Comment in
-
USP7 saves RIDDLE for the end.Cell Cycle. 2015;14(13):1999. doi: 10.1080/15384101.2015.1049087. Epub 2015 May 27. Cell Cycle. 2015. PMID: 26017280 Free PMC article. No abstract available.
Similar articles
-
USP14 regulates DNA damage repair by _targeting RNF168-dependent ubiquitination.Autophagy. 2018;14(11):1976-1990. doi: 10.1080/15548627.2018.1496877. Epub 2018 Aug 10. Autophagy. 2018. PMID: 29995557 Free PMC article.
-
An RNF168 fragment defective for focal accumulation at DNA damage is proficient for inhibition of homologous recombination in BRCA1 deficient cells.Nucleic Acids Res. 2014 Jul;42(12):7720-33. doi: 10.1093/nar/gku421. Epub 2014 May 14. Nucleic Acids Res. 2014. PMID: 24829461 Free PMC article.
-
USP3 counteracts RNF168 via deubiquitinating H2A and γH2AX at lysine 13 and 15.Cell Cycle. 2014;13(1):106-14. doi: 10.4161/cc.26814. Epub 2013 Oct 24. Cell Cycle. 2014. PMID: 24196443 Free PMC article.
-
RNF8-dependent histone ubiquitination during DNA damage response and spermatogenesis.Acta Biochim Biophys Sin (Shanghai). 2011 May;43(5):339-45. doi: 10.1093/abbs/gmr016. Epub 2011 Mar 28. Acta Biochim Biophys Sin (Shanghai). 2011. PMID: 21444325 Free PMC article. Review.
-
New answers to the old RIDDLE: RNF168 and the DNA damage response pathway.FEBS J. 2022 May;289(9):2467-2480. doi: 10.1111/febs.15857. Epub 2021 Apr 16. FEBS J. 2022. PMID: 33797206 Free PMC article. Review.
Cited by
-
E3 ligases: a ubiquitous link between DNA repair, DNA replication and human disease.Biochem J. 2024 Jul 17;481(14):923-944. doi: 10.1042/BCJ20240124. Biochem J. 2024. PMID: 38985307 Free PMC article. Review.
-
Human CRL4DDB2 ubiquitin ligase preferentially regulates post-repair chromatin restoration of H3K56Ac through recruitment of histone chaperon CAF-1.Onco_target. 2017 Oct 17;8(61):104525-104542. doi: 10.18632/onco_target.21869. eCollection 2017 Nov 28. Onco_target. 2017. PMID: 29262658 Free PMC article.
-
Stabilization of histone demethylase PHF8 by USP7 promotes breast carcinogenesis.J Clin Invest. 2016 Jun 1;126(6):2205-20. doi: 10.1172/JCI85747. Epub 2016 May 16. J Clin Invest. 2016. PMID: 27183383 Free PMC article.
-
Highlights in USP7 inhibitors for cancer treatment.Front Chem. 2022 Sep 15;10:1005727. doi: 10.3389/fchem.2022.1005727. eCollection 2022. Front Chem. 2022. PMID: 36186590 Free PMC article. Review.
-
Ubiquitin-Specific Peptidase 7: A Novel Deubiquitinase That Regulates Protein Homeostasis and Cancers.Front Oncol. 2021 Nov 19;11:784672. doi: 10.3389/fonc.2021.784672. eCollection 2021. Front Oncol. 2021. PMID: 34869041 Free PMC article. Review.
References
-
- Jackson SP, Bartek J. The DNA-damage response in human biology and disease. Nature 2009; 461:1071-8; PMID:19847258; http://dx.doi.org/10.1038/nature08467 - DOI - PMC - PubMed
-
- Harper JW, Elledge SJ. The DNA damage response: ten years after. Mol Cell 2007; 28:739-45; PMID:18082599; http://dx.doi.org/10.1016/j.molcel.2007.11.015 - DOI - PubMed
-
- Celeste A, Fernandez-Capetillo O, Kruhlak MJ, Pilch DR, Staudt DW, Lee A, Bonner RF, Bonner WM, Nussenzweig A. Histone H2AX phosphorylation is dispensable for the initial recognition of DNA breaks. Nat Cell Biol 2003; 5:675-9; PMID:12792649; http://dx.doi.org/10.1038/ncb1004 - DOI - PubMed
-
- Stucki M, Clapperton JA, Mohammad D, Yaffe MB, Smerdon SJ. and Jackson SP. MDC1 directly binds phosphorylated histone H2AX to regulate cellular responses to DNA double-strand breaks. Cell 2005; 123:1213-26; PMID:16377563; http://dx.doi.org/10.1016/j.cell.2005.09.038 - DOI - PubMed
-
- Lukas C, Falck J, Bartkova J, Bartek J, Lukas J. Distinct spatiotemporal dynamics of mammalian checkpoint regulators induced by DNA damage. Nat Cell Biol 2003; 5:255-60; PMID:12598907; http://dx.doi.org/10.1038/ncb945 - DOI - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous
