Patient-level meta-analysis of the EDITION 1, 2 and 3 studies: glycaemic control and hypoglycaemia with new insulin glargine 300 U/ml versus glargine 100 U/ml in people with type 2 diabetes
- PMID: 25929311
- PMCID: PMC4676914
- DOI: 10.1111/dom.12485
Patient-level meta-analysis of the EDITION 1, 2 and 3 studies: glycaemic control and hypoglycaemia with new insulin glargine 300 U/ml versus glargine 100 U/ml in people with type 2 diabetes
Abstract
Aims: To conduct a patient-level meta-analysis of the EDITION 1, 2 and 3 studies, which compared the efficacy and safety of new insulin glargine 300 U/ml (Gla-300) with insulin glargine 100 U/ml (Gla-100) in people with type 2 diabetes (T2DM) on basal and mealtime insulin, basal insulin and oral antihyperglycaemic drugs, or no prior insulin, respectively.
Methods: The EDITION studies were multicentre, randomized, open-label, parallel-group, phase IIIa studies, with similar designs and endpoints. A patient-level meta-analysis of the studies enabled these endpoints to be examined over 6 months in a large population with T2DM (Gla-300, n = 1247; Gla-100, n = 1249).
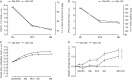
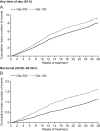
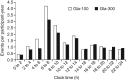
Results: No significant study-by-treatment interactions across studies were found, enabling them to be pooled. The mean change in glycated haemoglobin was comparable for Gla-300 and Gla-100 [each -1.02 (standard error 0.03)%; least squares (LS) mean difference 0.00 (95% confidence interval (CI) -0.08 to 0.07)%]. Annualized rates of confirmed (≤3.9 mmol/l) or severe hypoglycaemia were lower with Gla-300 than with Gla-100 during the night (31% difference in rate ratio over 6 months) and at any time (24 h, 14% difference). Consistent reductions were observed in percentage of participants with ≥1 hypoglycaemic event. Severe hypoglycaemia at any time (24 h) was rare (Gla-300: 2.3%; Gla-100: 2.6%). Weight gain was low (<1 kg) in both groups, with less gain with Gla-300 [LS mean difference -0.28 kg (95% CI -0.55 to -0.01); p = 0.039]. Both treatments were well tolerated, with similar rates of adverse events.
Conclusion: Gla-300 provides comparable glycaemic control to Gla-100 in a large population with a broad clinical spectrum of T2DM, with consistently less hypoglycaemia at any time of day and less nocturnal hypoglycaemia.
Keywords: basal insulin; insulin glargine; insulin therapy.
© 2015 The Authors. Diabetes, Obesity and Metabolism published by John Wiley & Sons Ltd.
Figures

Similar articles
-
New insulin glargine 300 U/ml versus glargine 100 U/ml in Japanese people with type 2 diabetes using basal insulin and oral antihyperglycaemic drugs: glucose control and hypoglycaemia in a randomized controlled trial (EDITION JP 2).Diabetes Obes Metab. 2016 Apr;18(4):366-74. doi: 10.1111/dom.12618. Epub 2016 Jan 21. Diabetes Obes Metab. 2016. PMID: 26662838 Free PMC article. Clinical Trial.
-
One-year sustained glycaemic control and less hypoglycaemia with new insulin glargine 300 U/ml compared with 100 U/ml in people with type 2 diabetes using basal plus meal-time insulin: the EDITION 1 12-month randomized trial, including 6-month extension.Diabetes Obes Metab. 2015 Sep;17(9):835-42. doi: 10.1111/dom.12472. Epub 2015 May 11. Diabetes Obes Metab. 2015. PMID: 25846721 Free PMC article. Clinical Trial.
-
Better glycaemic control and less hypoglycaemia with insulin glargine 300 U/mL vs glargine 100 U/mL: 1-year patient-level meta-analysis of the EDITION clinical studies in people with type 2 diabetes.Diabetes Obes Metab. 2018 Mar;20(3):541-548. doi: 10.1111/dom.13105. Epub 2017 Oct 5. Diabetes Obes Metab. 2018. PMID: 28862801 Free PMC article. Review.
-
Glycaemic control and hypoglycaemia with new insulin glargine 300 U/ml versus insulin glargine 100 U/ml in people with type 2 diabetes using basal insulin and oral antihyperglycaemic drugs: the EDITION 2 randomized 12-month trial including 6-month extension.Diabetes Obes Metab. 2015 Dec;17(12):1142-9. doi: 10.1111/dom.12532. Epub 2015 Sep 14. Diabetes Obes Metab. 2015. PMID: 26172084 Free PMC article. Clinical Trial.
-
A review of the safety and efficacy data for insulin glargine 300 units/ml, a new formulation of insulin glargine.Diabetes Obes Metab. 2015 Dec;17(12):1107-14. doi: 10.1111/dom.12531. Epub 2015 Sep 10. Diabetes Obes Metab. 2015. PMID: 26139151 Review.
Cited by
-
Impact of the mode of protraction of basal insulin therapies on their pharmacokinetic and pharmacodynamic properties and resulting clinical outcomes.Diabetes Obes Metab. 2017 Jan;19(1):3-12. doi: 10.1111/dom.12782. Epub 2016 Sep 26. Diabetes Obes Metab. 2017. PMID: 27593206 Free PMC article. Review.
-
Iatrogenic hypoglycemia-related hospital admissions identified through databases: economic burden and causes.Int J Clin Pharm. 2019 Oct;41(5):1159-1165. doi: 10.1007/s11096-019-00877-5. Epub 2019 Jul 23. Int J Clin Pharm. 2019. PMID: 31338669
-
Clinical Use of Insulin Glargine 300 U/mL in Adults with Type 2 Diabetes: Hypothetical Case Studies.Diabetes Ther. 2022 May;13(5):913-930. doi: 10.1007/s13300-022-01247-7. Epub 2022 Mar 30. Diabetes Ther. 2022. PMID: 35355207 Free PMC article. Review.
-
Use of Insulin Glargine 100 U/mL for the Treatment of Type 2 Diabetes Mellitus in East Asians: A Review.Diabetes Ther. 2019 Jun;10(3):805-833. doi: 10.1007/s13300-019-0613-7. Epub 2019 Apr 24. Diabetes Ther. 2019. PMID: 31020538 Free PMC article. Review.
-
High Concentration Insulin.Indian J Endocrinol Metab. 2018 Jan-Feb;22(1):160-163. doi: 10.4103/ijem.IJEM_300_17. Indian J Endocrinol Metab. 2018. PMID: 29535954 Free PMC article.
References
-
- Polonsky KS. The past 200 years in diabetes. N Engl J Med. 2012;367:1332–1340. - PubMed
-
- Holman RR, Farmer AJ, Davies MJ, et al. Three-year efficacy of complex insulin regimens in type 2 diabetes. N Engl J Med. 2009;361:1736–1747. - PubMed
-
- Riddle MC, Bolli GB, Ziemen M, Muehlen-Bartmer I, Bizet F, Home PD. New insulin glargine 300 units/mL versus glargine 100 units/mL in people with type 2 diabetes using basal and mealtime insulin: glucose control and hypoglycemia in a 6-month randomized controlled trial (EDITION 1) Diabetes Care. 2014;37:2755–2762. - PubMed
-
- Yki-Jarvinen H, Bergenstal R, Ziemen M, et al. New insulin glargine 300 units/mL versus glargine 100 units/mL in people with type 2 diabetes using oral agents and basal insulin: glucose control and hypoglycemia in a 6-month randomized controlled trial (EDITION 2) Diabetes Care. 2014;37:3235–3243. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

