Endothelin and the glomerulus in chronic kidney disease
- PMID: 25966347
- PMCID: PMC4731878
- DOI: 10.1016/j.semnephrol.2015.02.005
Endothelin and the glomerulus in chronic kidney disease
Abstract
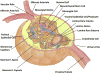
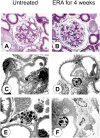
Endothelin-1 (ET-1) is a 21-amino acid peptide with mitogenic and powerful vasoconstricting properties. Under healthy conditions, ET-1 is expressed constitutively in all cells of the glomerulus and participates in homeostasis of glomerular structure and filtration function. Under disease conditions, increases in ET-1 are critically involved in initiating and maintaining glomerular inflammation, glomerular basement membrane hypertrophy, and injury of podocytes (visceral epithelial cells), thereby promoting proteinuria and glomerulosclerosis. Here, we review the role of ET-1 in the function of glomerular endothelial cells, visceral (podocytes) and parietal epithelial cells, mesangial cells, the glomerular basement membrane, stromal cells, inflammatory cells, and mesenchymal stem cells. We also discuss molecular mechanisms by which ET-1, predominantly through activation of the ETA receptor, contributes to injury to glomerular cells, and review preclinical and clinical evidence supporting its pathogenic role in glomerular injury in chronic renal disease. Finally, the therapeutic rationale for endothelin antagonists as a new class of antiproteinuric drugs is discussed.
Keywords: ERA; FSGS; GFR; albuminuria; blood pressure; chronic kidney disease; endothelin; endothelin receptor antagonists; epithelial cell; glomerulus; mesangial cells; podocyte; proteinuria.
Copyright © 2015 Elsevier Inc. All rights reserved.
Figures
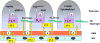

Similar articles
-
Actin dynamics at focal adhesions: a common endpoint and putative therapeutic _target for proteinuric kidney diseases.Kidney Int. 2018 Jun;93(6):1298-1307. doi: 10.1016/j.kint.2017.12.028. Epub 2018 Apr 17. Kidney Int. 2018. PMID: 29678354 Free PMC article. Review.
-
[The role of podocyte injury in chronic kidney disease].Nihon Rinsho Meneki Gakkai Kaishi. 2015;38(1):26-36. doi: 10.2177/jsci.38.26. Nihon Rinsho Meneki Gakkai Kaishi. 2015. PMID: 25765686 Review. Japanese.
-
Therapeutic potential of endothelin receptor antagonists for chronic proteinuric renal disease in humans.Biochim Biophys Acta. 2010 Dec;1802(12):1203-13. doi: 10.1016/j.bbadis.2010.03.012. Epub 2010 Mar 30. Biochim Biophys Acta. 2010. PMID: 20359530
-
Role of podocytes for reversal of glomerulosclerosis and proteinuria in the aging kidney after endothelin inhibition.Hypertension. 2004 Dec;44(6):974-81. doi: 10.1161/01.HYP.0000149249.09147.b4. Epub 2004 Nov 15. Hypertension. 2004. PMID: 15545511
-
Endothelin in nondiabetic chronic kidney disease: preclinical and clinical studies.Semin Nephrol. 2015 Mar;35(2):176-87. doi: 10.1016/j.semnephrol.2015.03.002. Semin Nephrol. 2015. PMID: 25966349 Review.
Cited by
-
Krüppel-like Factor 15: A Potential Therapeutic _target For Kidney Disease.Int J Biol Sci. 2019 Jul 21;15(9):1955-1961. doi: 10.7150/ijbs.34838. eCollection 2019. Int J Biol Sci. 2019. PMID: 31523196 Free PMC article. Review.
-
Sparsentan improves glomerular hemodynamics, cell functions, and tissue repair in a mouse model of FSGS.JCI Insight. 2024 Sep 3;9(19):e177775. doi: 10.1172/jci.insight.177775. JCI Insight. 2024. PMID: 39226116 Free PMC article.
-
Endothelin receptor antagonists in kidney protection for diabetic kidney disease and beyond?Nephrology (Carlton). 2023 Feb;28(2):97-108. doi: 10.1111/nep.14130. Epub 2022 Nov 15. Nephrology (Carlton). 2023. PMID: 36350038 Free PMC article. Review.
-
Tanshinone IIA Ameliorates Streptozotocin-Induced Diabetic Nephropathy, Partly by Attenuating PERK Pathway-Induced Fibrosis.Drug Des Devel Ther. 2020 Dec 31;14:5773-5782. doi: 10.2147/DDDT.S257734. eCollection 2020. Drug Des Devel Ther. 2020. PMID: 33408464 Free PMC article.
-
Drugs in Development to Treat IgA Nephropathy.Drugs. 2024 May;84(5):503-525. doi: 10.1007/s40265-024-02036-1. Epub 2024 May 23. Drugs. 2024. PMID: 38777962 Review.
References
-
- Barton M, Kohan DE. Endothelin in renal physiology and disease. 1. Karger AG; Basel: 2011. pp. 1–266.
-
- Benigni A. ET and diabetic nephropathy: pre-clinical and clinical studies. Sem Nephrol. 2015;35:188–196. - PubMed
-
- Schneider MP, Mann JF. Endothelin antagonism for patients with chronic kidney disease: still a hope for the future. Nephrol Dial Transplant. 2014;29(Suppl 1):i69–i73. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

