Inducible activation of ERK5 MAP kinase enhances adult neurogenesis in the olfactory bulb and improves olfactory function
- PMID: 25995470
- PMCID: PMC4438129
- DOI: 10.1523/JNEUROSCI.3745-14.2015
Inducible activation of ERK5 MAP kinase enhances adult neurogenesis in the olfactory bulb and improves olfactory function
Abstract
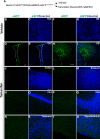
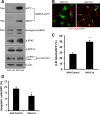
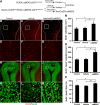
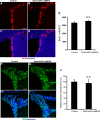
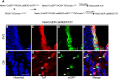
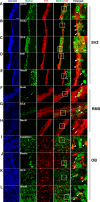
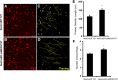
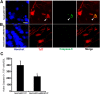
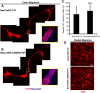
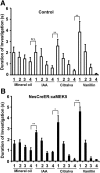
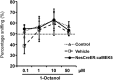
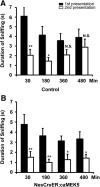
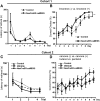
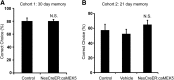
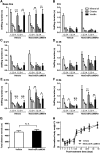
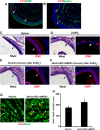
Recent discoveries have suggested that adult neurogenesis in the subventricular zone (SVZ) and olfactory bulb (OB) may be required for at least some forms of olfactory behavior in mice. However, it is unclear whether conditional and selective enhancement of adult neurogenesis by genetic approaches is sufficient to improve olfactory function under physiological conditions or after injury. Furthermore, specific signaling mechanisms regulating adult neurogenesis in the SVZ/OB are not fully defined. We previously reported that ERK5, a MAP kinase selectively expressed in the neurogenic regions of the adult brain, plays a critical role in adult neurogenesis in the SVZ/OB. Using a site-specific knock-in mouse model, we report here that inducible and _targeted activation of the endogenous ERK5 in adult neural stem/progenitor cells enhances adult neurogenesis in the OB by increasing cell survival and neuronal differentiation. This conditional ERK5 activation also improves short-term olfactory memory and odor-cued associative olfactory learning under normal physiological conditions. Furthermore, these mice show enhanced recovery of olfactory function and have more adult-born neurons after a zinc sulfate-induced lesion of the main olfactory epithelium. We conclude that ERK5 MAP kinase is an important endogenous signaling pathway regulating adult neurogenesis in the SVZ/OB, and that conditional activation of endogenous ERK5 is sufficient to enhance adult neurogenesis in the OB thereby improving olfactory function both under normal conditions and after injury.
Keywords: ERK5; MAP kinase; adult neurogenesis; olfaction.
Copyright © 2015 the authors 0270-6474/15/357833-17$15.00/0.
Figures
Similar articles
-
_targeted deletion of the ERK5 MAP kinase impairs neuronal differentiation, migration, and survival during adult neurogenesis in the olfactory bulb.PLoS One. 2013 Apr 22;8(4):e61948. doi: 10.1371/journal.pone.0061948. Print 2013. PLoS One. 2013. PMID: 23630619 Free PMC article.
-
Inducible and _targeted deletion of the ERK5 MAP kinase in adult neurogenic regions impairs adult neurogenesis in the olfactory bulb and several forms of olfactory behavior.PLoS One. 2012;7(11):e49622. doi: 10.1371/journal.pone.0049622. Epub 2012 Nov 21. PLoS One. 2012. PMID: 23185386 Free PMC article.
-
Inducible and conditional activation of ERK5 MAP kinase rescues mice from cadmium-induced olfactory memory deficits.Neurotoxicology. 2020 Dec;81:127-136. doi: 10.1016/j.neuro.2020.09.038. Epub 2020 Oct 8. Neurotoxicology. 2020. PMID: 33039505 Free PMC article.
-
Olfactory bulb neurogenesis depending on signaling in the subventricular zone.Cereb Cortex. 2023 Nov 4;33(22):11102-11111. doi: 10.1093/cercor/bhad349. Cereb Cortex. 2023. PMID: 37746807 Review.
-
The Adult Ventricular-Subventricular Zone (V-SVZ) and Olfactory Bulb (OB) Neurogenesis.Cold Spring Harb Perspect Biol. 2016 May 2;8(5):a018820. doi: 10.1101/cshperspect.a018820. Cold Spring Harb Perspect Biol. 2016. PMID: 27048191 Free PMC article. Review.
Cited by
-
Inducible and Conditional Stimulation of Adult Hippocampal Neurogenesis Rescues Cadmium-Induced Impairments of Adult Hippocampal Neurogenesis and Hippocampus-Dependent Memory in Mice.Toxicol Sci. 2020 Sep 1;177(1):263-280. doi: 10.1093/toxsci/kfaa104. Toxicol Sci. 2020. PMID: 32617577 Free PMC article.
-
[Progress in research on olfactory epithelial regeneration].Lin Chuang Er Bi Yan Hou Tou Jing Wai Ke Za Zhi. 2020 Apr;34(4):381-384. doi: 10.13201/j.issn.2096-7993.2020.04.024. Lin Chuang Er Bi Yan Hou Tou Jing Wai Ke Za Zhi. 2020. PMID: 32842240 Free PMC article. Review. Chinese.
-
Elastin-derived peptide VGVAPG decreases differentiation of mouse embryo fibroblast (3T3-L1) cells into adipocytes.Adipocyte. 2020 Dec;9(1):234-245. doi: 10.1080/21623945.2020.1770525. Adipocyte. 2020. PMID: 32463311 Free PMC article.
-
Chronic hyperglycemia regulates microglia polarization through ERK5.Aging (Albany NY). 2019 Jan 26;11(2):697-706. doi: 10.18632/aging.101770. Aging (Albany NY). 2019. PMID: 30684443 Free PMC article.
-
Adult Neurogenesis and Gliogenesis: Possible Mechanisms for Neurorestoration.Exp Neurobiol. 2016 Jun;25(3):103-12. doi: 10.5607/en.2016.25.3.103. Epub 2016 Jun 16. Exp Neurobiol. 2016. PMID: 27358578 Free PMC article. Review.
References
-
- Breton-Provencher V, Lemasson M, Peralta MR, 3rd, Saghatelyan A. Interneurons produced in adulthood are required for the normal functioning of the olfactory bulb network and for the execution of selected olfactory behaviors. J Neurosci. 2009;29:15245–15257. doi: 10.1523/JNEUROSCI.3606-09.2009. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous
