Nuclear lymphocyte-specific protein tyrosine kinase and its interaction with CR6-interacting factor 1 promote the survival of human leukemic T cells
- PMID: 25997448
- PMCID: PMC4484609
- DOI: 10.3892/or.2015.3990
Nuclear lymphocyte-specific protein tyrosine kinase and its interaction with CR6-interacting factor 1 promote the survival of human leukemic T cells
Abstract
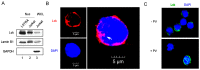
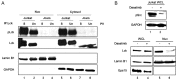
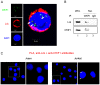
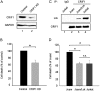
Overexpression and hyperactivation of lymphocyte-specific protein tyrosine kinase (Lck) have been associated with leukemia development. We previously showed that, other than its known function as a cytoplasmic signal transducer, Lck also acts as a nuclear transcription factor in mouse leukemic cells. In the present study, we demonstrated the presence of nuclear Lck in human leukemic T cells and in primary cells. We further established a positive correlation between Lck nuclear localization and its kinase activity. Proteomic analysis identified CR6-interacting factor 1 (CRIF1) as one of the Lck-interacting proteins. CRIF1 and Lck association in the nucleus was confirmed both by immunofluorescence microscopy and co-immunoprecipitation in human leukemic T cells. Close-range interaction between Lck and CRIF1 was validated by in situ proximity ligation assay (PLA). Consistent with the role of nuclear CRIF1 as a tumor suppressor, CRIF1 silencing promotes leukemic T cell survival in the absence of growth factors. This protective effect can be recapitulated by endogenous Lck or reconstituted Lck in leukemic T cells. All together, our results support a novel function of nuclear Lck in promoting human leukemic T cell survival through interaction with a tumor suppressor. It has important implications in defining a paradigm shift of non-canonical protein tyrosine kinase signaling.
Figures

Similar articles
-
Lymphocyte-specific protein tyrosine kinase (Lck) interacts with CR6-interacting factor 1 (CRIF1) in mitochondria to repress oxidative phosphorylation.BMC Cancer. 2015 Jul 26;15:551. doi: 10.1186/s12885-015-1520-6. BMC Cancer. 2015. PMID: 26210498 Free PMC article.
-
Functional interaction of the Ras effector RASSF5 with the tyrosine kinase Lck: critical role in nucleocytoplasmic transport and cell cycle regulation.J Mol Biol. 2010 Mar 19;397(1):89-109. doi: 10.1016/j.jmb.2010.01.005. Epub 2010 Jan 11. J Mol Biol. 2010. PMID: 20064523
-
In human leukemia cells ephrin-B-induced invasive activity is supported by Lck and is associated with reassembling of lipid raft signaling complexes.Mol Cancer Res. 2008 Feb;6(2):291-305. doi: 10.1158/1541-7786.MCR-07-0047. Mol Cancer Res. 2008. PMID: 18314490
-
A novel link between Lck, Bak expression and chemosensitivity.Oncogene. 2006 Mar 16;25(12):1693-5. doi: 10.1038/sj.onc.1209157. Oncogene. 2006. PMID: 16186791 Review.
-
Multifunctions of CRIF1 in cancers and mitochondrial dysfunction.Front Oncol. 2022 Oct 3;12:1009948. doi: 10.3389/fonc.2022.1009948. eCollection 2022. Front Oncol. 2022. PMID: 36263222 Free PMC article. Review.
Cited by
-
Lck Function and Modulation: Immune Cytotoxic Response and Tumor Treatment More Than a Simple Event.Cancers (Basel). 2024 Jul 24;16(15):2630. doi: 10.3390/cancers16152630. Cancers (Basel). 2024. PMID: 39123358 Free PMC article. Review.
-
Unlocking the potential of T-cell metabolism reprogramming: Advancing single-cell approaches for precision immunotherapy in tumour immunity.Clin Transl Med. 2024 Mar;14(3):e1620. doi: 10.1002/ctm2.1620. Clin Transl Med. 2024. PMID: 38468489 Free PMC article. Review.
-
Mitochondrial ribosomes in cancer.Semin Cancer Biol. 2017 Dec;47:67-81. doi: 10.1016/j.semcancer.2017.04.004. Epub 2017 Apr 23. Semin Cancer Biol. 2017. PMID: 28445780 Free PMC article. Review.
-
Unraveling the roles and mechanisms of mitochondrial translation in normal and malignant hematopoiesis.J Hematol Oncol. 2024 Oct 12;17(1):95. doi: 10.1186/s13045-024-01615-9. J Hematol Oncol. 2024. PMID: 39396039 Free PMC article. Review.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Miscellaneous

