Physiologically-based pharmacokinetic modeling of renally excreted antiretroviral drugs in pregnant women
- PMID: 26011128
- PMCID: PMC4631176
- DOI: 10.1111/bcp.12685
Physiologically-based pharmacokinetic modeling of renally excreted antiretroviral drugs in pregnant women
Abstract
Aim: Physiological changes during pregnancy can affect drug disposition. Anticipating these changes will help to maximize drug efficacy and safety in pregnant women. Our objective was to determine if physiologically-based pharmacokinetics (PBPK) can accurately predict changes in the disposition of renally excreted antiretroviral drugs during pregnancy.
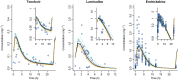
Methods: Whole body PBPK models were developed for three renally excreted antiretroviral drugs, tenofovir (TFV), emtricitabine (FTC) and lamivudine (3TC). To assess the impact of pregnancy on PK, time-varying pregnancy-related physiological parameters available within the p-PBPK Simcyp software package were used. Renal clearance during pregnancy followed glomerular filtration changes with or without alterations in secretion. PK profiles were simulated and compared with observed data, i.e. area under the curves (AUC), peak plasma concentrations (Cmax ) and oral clearances (CL/F).
Results: PBPK models successfully predicted TFV, FTC and 3TC disposition for non-pregnant and pregnant populations. Both renal secretion and filtration changed during pregnancy. Changes in renal clearance secretion were related to changes in renal plasma flow. The maximum clearance increases were approximately 30% (TFV 33%, FTC 31%, 3TC 29%).
Conclusions: Pregnancy PBPK models are useful tools to quantify a priori the drug exposure changes during pregnancy for renally excreted drugs. These models can be applied to evaluate alternative dosing regimens to optimize drug therapy during pregnancy.
Keywords: PBPK; emtricitabine; lamivudine; pharmacokinetics; pregnancy; tenofovir.
© 2015 The British Pharmacological Society.
Figures
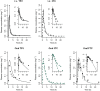
 TFV observed (Deeks et al. 28),
TFV observed (Deeks et al. 28),  3TC observed (Johnson et al. 21),
3TC observed (Johnson et al. 21),  TFV observed (Wenning et al. 39),
TFV observed (Wenning et al. 39),  3TC observed (Wang et al. 38),
3TC observed (Wang et al. 38),  FTC observed,
FTC observed,  TFV predicted,
TFV predicted,  3TC predicted,
3TC predicted,  TFV predicted,
TFV predicted,  3TC predicted,
3TC predicted,  FTC predicted
FTC predicted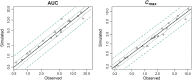
 3TC,
3TC,  FTC,
FTC,  Line of unity,
Line of unity,  1.25-fold,
1.25-fold,  2-fold
2-fold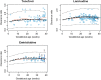
 mean predicted clearance ratio (GFR),
mean predicted clearance ratio (GFR),  mean predicted clearance ratio (GFR + renal blood flow),
mean predicted clearance ratio (GFR + renal blood flow),  predicted IP,
predicted IP,  spline,
spline,  population PK data, 1 Data form Colbers et al. , 2 Data from Stek et al. , 3 Data from Hirt et al.
population PK data, 1 Data form Colbers et al. , 2 Data from Stek et al. , 3 Data from Hirt et al.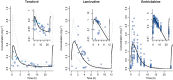
Similar articles
-
Physiologically Based Pharmacokinetic Models to Predict Maternal Pharmacokinetics and Fetal Exposure to Emtricitabine and Acyclovir.J Clin Pharmacol. 2020 Feb;60(2):240-255. doi: 10.1002/jcph.1515. Epub 2019 Sep 6. J Clin Pharmacol. 2020. PMID: 31489678 Free PMC article.
-
Are Standard Doses of Renally-Excreted Antiretrovirals in Older Patients Appropriate: A PBPK Study Comparing Exposures in the Elderly Population With Those in Renal Impairment.Drugs R D. 2019 Dec;19(4):339-350. doi: 10.1007/s40268-019-00285-0. Drugs R D. 2019. PMID: 31602556 Free PMC article.
-
Physiologically Based Pharmacokinetic Modeling of Renally Cleared Drugs in Pregnant Women.Clin Pharmacokinet. 2017 Dec;56(12):1525-1541. doi: 10.1007/s40262-017-0538-0. Clin Pharmacokinet. 2017. PMID: 28391404
-
Tenofovir disoproxil fumarate-emtricitabine coformulation for once-daily dual NRTI backbone.Expert Rev Anti Infect Ther. 2006 Aug;4(4):523-35. doi: 10.1586/14787210.4.4.523. Expert Rev Anti Infect Ther. 2006. PMID: 17009933 Review.
-
Pharmacometrics in pregnancy: An unmet need.Annu Rev Pharmacol Toxicol. 2014;54:53-69. doi: 10.1146/annurev-pharmtox-011613-140009. Annu Rev Pharmacol Toxicol. 2014. PMID: 24392692 Review.
Cited by
-
An update on placental drug transport and its relevance to fetal drug exposure.Med Rev (2021). 2022 Nov 21;2(5):501-511. doi: 10.1515/mr-2022-0025. eCollection 2022 Oct. Med Rev (2021). 2022. PMID: 37724167 Free PMC article. Review.
-
Pharmacokinetics of the most commonly used antihypertensive drugs throughout pregnancy methyldopa, labetalol, and nifedipine: a systematic review.Eur J Clin Pharmacol. 2022 Nov;78(11):1763-1776. doi: 10.1007/s00228-022-03382-3. Epub 2022 Sep 15. Eur J Clin Pharmacol. 2022. PMID: 36104450 Free PMC article. Review.
-
Safety and Efficacy of Antiviral Drugs and Vaccines in Pregnant Women: Insights from Physiologically Based Pharmacokinetic Modeling and Integration of Viral Infection Dynamics.Vaccines (Basel). 2024 Jul 16;12(7):782. doi: 10.3390/vaccines12070782. Vaccines (Basel). 2024. PMID: 39066420 Free PMC article. Review.
-
Lamivudine and Emtricitabine Dosing Proposal for Children with HIV and Chronic Kidney Disease, Supported by Physiologically Based Pharmacokinetic Modelling.Pharmaceutics. 2023 May 6;15(5):1424. doi: 10.3390/pharmaceutics15051424. Pharmaceutics. 2023. PMID: 37242665 Free PMC article.
-
Therapeutic levetiracetam monitoring during pregnancy: "mind the gap".Ther Adv Chronic Dis. 2019 May 27;10:2040622319851652. doi: 10.1177/2040622319851652. eCollection 2019. Ther Adv Chronic Dis. 2019. PMID: 31191874 Free PMC article.
References
-
- UNAIDS. 2013. UNAIDS 2013 [Internet]. Global report 2013.. Available at http://www.unaids.org/en/resources/documents/2013/ (last accessed 17 December 2013)
-
- 2013. WHO | Guidelines: HIV [Internet]. WHO.. Available at http://www.who.int/hiv/pub/guidelines/en/ (last accessed 30 July 2014)
-
- Ke AB, Rostami-Hodjegan A, Zhao P, Unadkat JD. Pharmacometrics in pregnancy: an unmet need. Annu Rev Pharmacol Toxicol. 2014;54:53–69. - PubMed
-
- Anderson GD. Pregnancy-induced changes in pharmacokinetics: a mechanistic-based approach. Clin Pharmacokinet. 2005;44:989–1008. - PubMed
-
- Luecke RH, Wosilait WD, Pearce BA, Young JF. A computer model and program for xenobiotic disposition during pregnancy. Comput Methods Programs Biomed. 1997;53:201–24. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

