Inhibition of Aerobic Glycolysis Represses Akt/mTOR/HIF-1α Axis and Restores Tamoxifen Sensitivity in Antiestrogen-Resistant Breast Cancer Cells
- PMID: 26158266
- PMCID: PMC4497721
- DOI: 10.1371/journal.pone.0132285
Inhibition of Aerobic Glycolysis Represses Akt/mTOR/HIF-1α Axis and Restores Tamoxifen Sensitivity in Antiestrogen-Resistant Breast Cancer Cells
Abstract
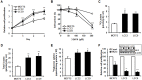
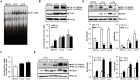
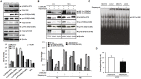
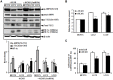
Tamoxifen resistance is often observed in the majority of estrogen receptor-positive breast cancers and it remains as a serious clinical problem in breast cancer management. Increased aerobic glycolysis has been proposed as one of the mechanisms for acquired resistance to chemotherapeutic agents in breast cancer cells such as adriamycin. Herein, we report that the glycolysis rates in LCC2 and LCC9--tamoxifen-resistant human breast cancer cell lines derived from MCF7--are higher than those in MCF7S, which is the parent MCF7 subline. Inhibition of key glycolytic enzyme such as hexokinase-2 resulted in cell growth retardation at higher degree in LCC2 and LCC9 than that in MCF7S. This implies that increased aerobic glycolysis even under O2-rich conditions, a phenomenon known as the Warburg effect, is closely associated with tamoxifen resistance. We found that HIF-1α is activated via an Akt/mTOR signaling pathway in LCC2 and LCC9 cells without hypoxic condition. Importantly, specific inhibition of hexokinase-2 suppressed the activity of Akt/mTOR/HIF-1α axis in LCC2 and LCC9 cells. In addition, the phosphorylated AMPK which is a negative regulator of mTOR was decreased in LCC2 and LCC9 cells compared to MCF7S. Interestingly, either the inhibition of mTOR activity or increase in AMPK activity induced a reduction in lactate accumulation and cell survival in the LCC2 and LCC9 cells. Taken together, our data provide evidence that development of tamoxifen resistance may be driven by HIF-1α hyperactivation via modulation of Akt/mTOR and/or AMPK signaling pathways. Therefore, we suggest that the HIF-1α hyperactivation is a critical marker of increased aerobic glycolysis in accordance with tamoxifen resistance and thus restoration of aerobic glycolysis may be novel therapeutic _target for treatment of tamoxifen-resistant breast cancer.
Conflict of interest statement
Figures

Similar articles
-
Knockdown of KLF5 suppresses hypoxia-induced resistance to cisplatin in NSCLC cells by regulating HIF-1α-dependent glycolysis through inactivation of the PI3K/Akt/mTOR pathway.J Transl Med. 2018 Jun 14;16(1):164. doi: 10.1186/s12967-018-1543-2. J Transl Med. 2018. PMID: 29898734 Free PMC article.
-
Long Non-Coding RNA (lncRNA) Urothelial Carcinoma-Associated 1 (UCA1) Enhances Tamoxifen Resistance in Breast Cancer Cells via Inhibiting mTOR Signaling Pathway.Med Sci Monit. 2016 Oct 21;22:3860-3867. doi: 10.12659/msm.900689. Med Sci Monit. 2016. PMID: 27765938 Free PMC article.
-
Inhibition of mTOR activity restores tamoxifen response in breast cancer cells with aberrant Akt Activity.Clin Cancer Res. 2004 Dec 1;10(23):8059-67. doi: 10.1158/1078-0432.CCR-04-0035. Clin Cancer Res. 2004. PMID: 15585641
-
Metabolic phenotype of bladder cancer.Cancer Treat Rev. 2016 Apr;45:46-57. doi: 10.1016/j.ctrv.2016.03.005. Epub 2016 Mar 8. Cancer Treat Rev. 2016. PMID: 26975021 Review.
-
The sweet trap in tumors: aerobic glycolysis and potential _targets for therapy.Onco_target. 2016 Jun 21;7(25):38908-38926. doi: 10.18632/onco_target.7676. Onco_target. 2016. PMID: 26918353 Free PMC article. Review.
Cited by
-
Tumor Cell Glycolysis-At the Crossroad of Epithelial-Mesenchymal Transition and Autophagy.Cells. 2022 Mar 18;11(6):1041. doi: 10.3390/cells11061041. Cells. 2022. PMID: 35326492 Free PMC article. Review.
-
Inhibition of Metabolism as a Therapeutic Option for Tamoxifen-Resistant Breast Cancer Cells.Cells. 2021 Sep 12;10(9):2398. doi: 10.3390/cells10092398. Cells. 2021. PMID: 34572047 Free PMC article.
-
Extracellular matrix protein Reelin promotes myeloma progression by facilitating tumor cell proliferation and glycolysis.Sci Rep. 2017 Mar 27;7:45305. doi: 10.1038/srep45305. Sci Rep. 2017. PMID: 28345605 Free PMC article.
-
EDIL3 alleviates Mannan-induced psoriatic arthritis by slowing the intracellular glycolysis process in mononuclear-derived dendritic cells.Inflammation. 2024 Sep 18. doi: 10.1007/s10753-024-02134-y. Online ahead of print. Inflammation. 2024. PMID: 39289212
-
Phosphoserine aminotransferase 1 is associated to poor outcome on tamoxifen therapy in recurrent breast cancer.Sci Rep. 2017 May 18;7(1):2099. doi: 10.1038/s41598-017-02296-w. Sci Rep. 2017. PMID: 28522855 Free PMC article.
References
-
- Dodwell D, Williamson D (2008) Beyond tamoxifen: extended and late extended endocrine therapy in postmenopausal early breast cancer. Cancer Treat Rev 34: 137–144. - PubMed
-
- Cigler T, Goss PE (2007) Breast cancer adjuvant endocrine therapy. Cancer J 13: 148–155. - PubMed
-
- Fan P, Wang J, Santen RJ, Yue W (2007) Long-term treatment with tamoxifen facilitates translocation of estrogen receptor alpha out of the nucleus and enhances its interaction with EGFR in MCF-7 breast cancer cells. Cancer Res 67: 1352–1360. - PubMed
-
- Neeman M, Degani H (1989) Metabolic studies of estrogen- and tamoxifen-treated human breast cancer cells by nuclear magnetic resonance spectroscopy. Cancer Res 49: 589–594. - PubMed
-
- Rivenzon-Segal D, Boldin-Adamsky S, Seger D, Seger R, Degani H (2003) Glycolysis and glucose transporter 1 as markers of response to hormonal therapy in breast cancer. Int J Cancer 107: 177–182. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

