Glycaemic control and hypoglycaemia with new insulin glargine 300 U/ml versus insulin glargine 100 U/ml in people with type 2 diabetes using basal insulin and oral antihyperglycaemic drugs: the EDITION 2 randomized 12-month trial including 6-month extension
- PMID: 26172084
- PMCID: PMC5049622
- DOI: 10.1111/dom.12532
Glycaemic control and hypoglycaemia with new insulin glargine 300 U/ml versus insulin glargine 100 U/ml in people with type 2 diabetes using basal insulin and oral antihyperglycaemic drugs: the EDITION 2 randomized 12-month trial including 6-month extension
Abstract
Aims: To compare the efficacy and safety of new insulin glargine 300 U/ml (Gla-300) with insulin glargine 100 U/ml (Gla-100) over 12 months of treatment in people with type 2 diabetes using basal insulin and oral antihyperglycaemic drugs (OADs).
Methods: EDITION 2 (NCT01499095) was a randomized, 6-month, multicentre, open-label, two-arm, phase IIIa study investigating once-daily Gla-300 versus Gla-100, plus OADs (excluding sulphonylureas), with a 6-month safety extension.
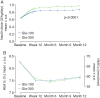
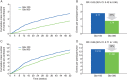
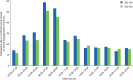
Results: Similar numbers of participants in each group completed 12 months of treatment [Gla-300, 315 participants (78%); Gla-100, 314 participants (77%)]. The reduction in glycated haemoglobin was maintained for 12 months with both treatments: least squares (LS) mean (standard error) change from baseline -0.55 (0.06)% for Gla-300 and -0.50 (0.06)% for Gla-100; LS mean difference -0.06 [95% confidence interval (CI) -0.22 to 0.10)%]. A significant relative reduction of 37% in the annualized rate of nocturnal confirmed [≤3.9 mmol/l (≤70 mg/dl)] or severe hypoglycaemia was observed with Gla-300 compared with Gla-100: rate ratio 0.63 [(95% CI 0.42-0.96); p = 0.031], and fewer participants experienced ≥1 event [relative risk 0.84 (95% CI 0.71-0.99)]. Severe hypoglycaemia was infrequent. Weight gain was significantly lower with Gla-300 than Gla-100 [LS mean difference -0.7 (95% CI -1.3 to -0.2) kg; p = 0.009]. Both treatments were well tolerated with a similar pattern of adverse events (incidence of 69 and 60% in the Gla-300 and Gla-100 groups).
Conclusions: In people with type 2 diabetes treated with Gla-300 or Gla-100, and non-sulphonylurea OADs, glycaemic control was sustained over 12 months, with less nocturnal hypoglycaemia in the Gla-300 group.
Keywords: basal insulin; insulin glargine; oral antihyperglycaemic drugs; type 2 diabetes.
© 2015 The Authors. Diabetes, Obesity and Metabolism published by John Wiley & Sons Ltd.
Figures
Similar articles
-
New insulin glargine 300 U/ml versus glargine 100 U/ml in Japanese people with type 2 diabetes using basal insulin and oral antihyperglycaemic drugs: glucose control and hypoglycaemia in a randomized controlled trial (EDITION JP 2).Diabetes Obes Metab. 2016 Apr;18(4):366-74. doi: 10.1111/dom.12618. Epub 2016 Jan 21. Diabetes Obes Metab. 2016. PMID: 26662838 Free PMC article. Clinical Trial.
-
New insulin glargine 300 U/ml compared with glargine 100 U/ml in insulin-naïve people with type 2 diabetes on oral glucose-lowering drugs: a randomized controlled trial (EDITION 3).Diabetes Obes Metab. 2015 Apr;17(4):386-94. doi: 10.1111/dom.12438. Epub 2015 Feb 12. Diabetes Obes Metab. 2015. PMID: 25641260 Free PMC article. Clinical Trial.
-
Glycaemic control and hypoglycaemia in people with type 2 diabetes switching from twice-daily basal insulin to once-daily insulin glargine 300 U/mL or insulin glargine 100 U/mL (EDITION 1 and EDITION 2 subgroup analysis).Diabetes Obes Metab. 2018 Feb;20(2):448-452. doi: 10.1111/dom.13071. Epub 2017 Sep 14. Diabetes Obes Metab. 2018. PMID: 28736942 Free PMC article. Clinical Trial.
-
A review of the safety and efficacy data for insulin glargine 300 units/ml, a new formulation of insulin glargine.Diabetes Obes Metab. 2015 Dec;17(12):1107-14. doi: 10.1111/dom.12531. Epub 2015 Sep 10. Diabetes Obes Metab. 2015. PMID: 26139151 Review.
-
Better glycaemic control and less hypoglycaemia with insulin glargine 300 U/mL vs glargine 100 U/mL: 1-year patient-level meta-analysis of the EDITION clinical studies in people with type 2 diabetes.Diabetes Obes Metab. 2018 Mar;20(3):541-548. doi: 10.1111/dom.13105. Epub 2017 Oct 5. Diabetes Obes Metab. 2018. PMID: 28862801 Free PMC article. Review.
Cited by
-
Efficacy and safety of insulin glargine 300 U/mL (Gla-300) during hospitalization and therapy intensification at discharge in patients with insufficiently controlled type 2 diabetes: results of the phase IV COBALTA trial.BMJ Open Diabetes Res Care. 2020 Sep;8(1):e001518. doi: 10.1136/bmjdrc-2020-001518. BMJ Open Diabetes Res Care. 2020. PMID: 32928792 Free PMC article. Clinical Trial.
-
Improved Glycemic Control with Insulin Glargine 300 U/mL (Toujeo®) in Patients with Type 2 Diabetes: Real-World Effectiveness in Switzerland.Diabetes Ther. 2018 Dec;9(6):2325-2334. doi: 10.1007/s13300-018-0518-x. Epub 2018 Oct 9. Diabetes Ther. 2018. PMID: 30302721 Free PMC article.
-
Differential glycaemic control with basal insulin glargine 300 U/mL versus degludec 100 U/mL according to kidney function in type 2 diabetes: A subanalysis from the BRIGHT trial.Diabetes Obes Metab. 2020 Aug;22(8):1369-1377. doi: 10.1111/dom.14043. Epub 2020 Apr 28. Diabetes Obes Metab. 2020. PMID: 32243043 Free PMC article. Clinical Trial.
-
Risk of hypoglycaemia with insulin degludec versus insulin glargine U300 in insulin-treated patients with type 2 diabetes: the randomised, head-to-head CONCLUDE trial.Diabetologia. 2020 Apr;63(4):698-710. doi: 10.1007/s00125-019-05080-9. Epub 2020 Jan 27. Diabetologia. 2020. PMID: 31984443 Free PMC article. Clinical Trial.
-
[Insulin therapy-new insulin analogues].Internist (Berl). 2019 Sep;60(9):887-894. doi: 10.1007/s00108-019-0652-1. Internist (Berl). 2019. PMID: 31396651 Review. German.
References
-
- Kunt T, Snoek FJ. Barriers to insulin initiation and intensification and how to overcome them. Int J Clin Pract 2009; 63 (Suppl. 164): 6–10. - PubMed
-
- Yki‐Järvinen H, Bergenstal R, Ziemen M et al. New insulin glargine 300 U/mL versus glargine 100 U/mL in people with type 2 diabetes using oral agents and basal insulin: glucose control and hypoglycaemia in a 6‐month randomised controlled trial (EDITION 2). Diabetes Care 2014; 37: 3235–3243. - PubMed
-
- Bolli G, Riddle M, Bergenstal R et al. New insulin glargine 300 U/mL versus glargine 100 U/mL in insulin naïve people with type 2 diabetes using anithyperglycemic drugs: glucose control and hypoglycemia in a randomized controlled trial (EDITION 3). Diabetes Obes Metab 2015; 17: 386–394. - PMC - PubMed
-
- Riddle MC, Bolli GB, Ziemen M, Muehlen‐Bartmer I, Bizet F, Home PD. New insulin glargine 300 units/mL versus glargine 100 units/mL in people with type 2 diabetes using basal and mealtime insulin: glucose control and hypoglycemia in a 6‐month randomized controlled trial (EDITION 1). Diabetes Care 2014; 37: 2755–2762. - PubMed
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

