Treatment With Lopinavir/Ritonavir or Interferon-β1b Improves Outcome of MERS-CoV Infection in a Nonhuman Primate Model of Common Marmoset
- PMID: 26198719
- PMCID: PMC7107395
- DOI: 10.1093/infdis/jiv392
Treatment With Lopinavir/Ritonavir or Interferon-β1b Improves Outcome of MERS-CoV Infection in a Nonhuman Primate Model of Common Marmoset
Abstract
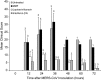
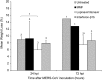
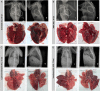
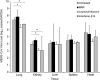
Middle East respiratory syndrome coronavirus (MERS-CoV) causes severe disease in human with an overall case-fatality rate of >35%. Effective antivirals are crucial for improving the clinical outcome of MERS. Although a number of repurposed drugs, convalescent-phase plasma, antiviral peptides, and neutralizing antibodies exhibit anti-MERS-CoV activity in vitro, most are not readily available or have not been evaluated in nonhuman primates. We assessed 3 repurposed drugs with potent in vitro anti-MERS-CoV activity (mycophenolate mofetil [MMF], lopinavir/ritonavir, and interferon-β1b) in common marmosets with severe disease resembling MERS in humans. The lopinavir/ritonavir-treated and interferon-β1b-treated animals had better outcome than the untreated animals, with improved clinical (mean clinical scores ↓50.9%-95.0% and ↓weight loss than the untreated animals), radiological (minimal pulmonary infiltrates), and pathological (mild bronchointerstitial pneumonia) findings, and lower mean viral loads in necropsied lung (↓0.59-1.06 log10 copies/glyceraldehyde 3-phosphate dehydrogenase [GAPDH]; P < .050) and extrapulmonary (↓0.11-1.29 log10 copies/GAPDH; P < .050 in kidney) tissues. In contrast, all MMF-treated animals developed severe and/or fatal disease with higher mean viral loads (↑0.15-0.54 log10 copies/GAPDH) than the untreated animals. The mortality rate at 36 hours postinoculation was 67% (untreated and MMF-treated) versus 0-33% (lopinavir/ritonavir-treated and interferon-β1b-treated). Lopinavir/ritonavir and interferon-β1b alone or in combination should be evaluated in clinical trials. MMF alone may worsen MERS and should not be used.
Keywords: Kaletra; MERS; animal; common marmoset; coronavirus; interferon; lopinavir; mycophenolate; primate; treatment.
© The Author 2015. Published by Oxford University Press on behalf of the Infectious Diseases Society of America. All rights reserved. For Permissions, please e-mail: journals.permissions@oup.com.
Figures


Similar articles
-
Treatment of Middle East Respiratory Syndrome with a combination of lopinavir-ritonavir and interferon-β1b (MIRACLE trial): study protocol for a randomized controlled trial.Trials. 2018 Jan 30;19(1):81. doi: 10.1186/s13063-017-2427-0. Trials. 2018. PMID: 29382391 Free PMC article.
-
Combination therapy with lopinavir/ritonavir, ribavirin and interferon-α for Middle East respiratory syndrome.Antivir Ther. 2016;21(5):455-9. doi: 10.3851/IMP3002. Epub 2015 Oct 22. Antivir Ther. 2016. PMID: 26492219
-
Prophylactic and therapeutic efficacy of mAb treatment against MERS-CoV in common marmosets.Antiviral Res. 2018 Aug;156:64-71. doi: 10.1016/j.antiviral.2018.06.006. Epub 2018 Jun 7. Antiviral Res. 2018. PMID: 29885377 Free PMC article.
-
A review of candidate therapies for Middle East respiratory syndrome from a molecular perspective.J Med Microbiol. 2017 Sep;66(9):1261-1274. doi: 10.1099/jmm.0.000565. Epub 2017 Aug 31. J Med Microbiol. 2017. PMID: 28855003 Free PMC article. Review.
-
A Comparative Review of Animal Models of Middle East Respiratory Syndrome Coronavirus Infection.Vet Pathol. 2016 May;53(3):521-31. doi: 10.1177/0300985815620845. Epub 2016 Feb 11. Vet Pathol. 2016. PMID: 26869154 Review.
Cited by
-
Pathophysiology and Potential Therapeutic Candidates for COVID-19: A Poorly Understood Arena.Front Pharmacol. 2020 Sep 17;11:585888. doi: 10.3389/fphar.2020.585888. eCollection 2020. Front Pharmacol. 2020. PMID: 33041830 Free PMC article. Review.
-
COVID-19 and immunological regulations - from basic and translational aspects to clinical implications.J Dtsch Dermatol Ges. 2020 Aug;18(8):795-807. doi: 10.1111/ddg.14169. Epub 2020 Aug 6. J Dtsch Dermatol Ges. 2020. PMID: 32761894 Free PMC article. Review.
-
Clinical characteristics and plasma antibody titer of patients with COVID-19 in Zhejiang, China.J Zhejiang Univ Sci B. 2020 Dec.;21(12):955-960. doi: 10.1631/jzus.B2000593. J Zhejiang Univ Sci B. 2020. PMID: 33843161 Free PMC article.
-
Comparative molecular investigation of the potential inhibitors against SARS-CoV-2 main protease: a molecular docking study.J Biomol Struct Dyn. 2021 Oct;39(16):6317-6323. doi: 10.1080/07391102.2020.1796813. Epub 2020 Jul 22. J Biomol Struct Dyn. 2021. PMID: 32696718 Free PMC article.
-
MERS-CoV spike protein: _targets for vaccines and therapeutics.Antiviral Res. 2016 Sep;133:165-77. doi: 10.1016/j.antiviral.2016.07.015. Epub 2016 Jul 26. Antiviral Res. 2016. PMID: 27468951 Free PMC article. Review.
References
-
- Zaki AM, van Boheemen S, Bestebroer TM, Osterhaus AD, Fouchier RA. Isolation of a novel coronavirus from a man with pneumonia in Saudi Arabia. N Engl J Med 2012; 367:1814–20. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

