Hypoxia-Inducible Factor-1: A Critical Player in the Survival Strategy of Stressed Cells
- PMID: 26206147
- PMCID: PMC4715696
- DOI: 10.1002/jcb.25283
Hypoxia-Inducible Factor-1: A Critical Player in the Survival Strategy of Stressed Cells
Abstract
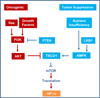
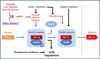
HIF-1 activation has been well known as an adaptive strategy to hypoxia. Recently it became clear that hypoxia was often accompanied by insufficient supply of glucose or amino acids as a common result of poor circulation that frequently occurs in solid tumors and ischemic lesions, creating a mixed nutrient insufficiency. In response to nutrient insufficiency, stressed cells elicit survival strategies including activation of AMPK and HIF-1 to cope with the stress. Particularly, in solid tumors, HIF-1 promotes cell survival and migration, stimulates angiogenesis, and induces resistance to radiation and chemotherapy. Interestingly, radiation and some chemotherapeutics are reported to trigger the activation of AMPK. Here we discuss the recent advances that may potentially link the stress responsive mechanisms including AMPK activation, ATF4 activation and the enhancement of Hsp70/Hsp90 function to HIF-1 activation. Potential implication and application of the stress-facilitated HIF-1 activation in solid tumors and ischemic disorders will be discussed. A better understanding of HIF-1 activation in cells exposed to stresses is expected to facilitate the design of therapeutic approaches that specifically modulate cell survival strategy.
Keywords: AMPK; HDAC4; HDAC5; HIF-1; HYPOXIA; Hsp70; Hsp90; STRESS RESPONSE.
© 2015 Wiley Periodicals, Inc.
Figures


Similar articles
-
AMPK-HDAC5 pathway facilitates nuclear accumulation of HIF-1α and functional activation of HIF-1 by deacetylating Hsp70 in the cytosol.Cell Cycle. 2015 Aug 3;14(15):2520-36. doi: 10.1080/15384101.2015.1055426. Epub 2015 Jun 10. Cell Cycle. 2015. PMID: 26061431 Free PMC article.
-
Transcriptional activation of hypoxia-inducible factor-1alpha by HDAC4 and HDAC5 involves differential recruitment of p300 and FIH-1.FEBS Lett. 2009 Jan 5;583(1):55-60. doi: 10.1016/j.febslet.2008.11.044. Epub 2008 Dec 9. FEBS Lett. 2009. PMID: 19071119
-
Functional activation of heat shock factor and hypoxia-inducible factor in the kidney.J Am Soc Nephrol. 2002 Aug;13(8):2094-101. doi: 10.1097/01.asn.0000022008.30175.5b. J Am Soc Nephrol. 2002. PMID: 12138141
-
HIF at the crossroads between ischemia and carcinogenesis.J Cell Physiol. 2004 Jul;200(1):20-30. doi: 10.1002/jcp.10479. J Cell Physiol. 2004. PMID: 15137054 Review.
-
Harnessing the hypoxia-inducible factor in cancer and ischemic disease.Biochem Pharmacol. 2007 Feb 1;73(3):450-7. doi: 10.1016/j.bcp.2006.10.013. Epub 2006 Oct 20. Biochem Pharmacol. 2007. PMID: 17101119 Review.
Cited by
-
Nicotinamide riboside, an NAD+ precursor, attenuates inflammation and oxidative stress by activating sirtuin 1 in alcohol-stimulated macrophages.Lab Invest. 2021 Sep;101(9):1225-1237. doi: 10.1038/s41374-021-00599-1. Epub 2021 Apr 12. Lab Invest. 2021. PMID: 33846538
-
Anti-seizure Effects and Mechanisms of Berberine: A Systematic Review.Curr Pharm Biotechnol. 2024;25(17):2253-2265. doi: 10.2174/0113892010283237240107121749. Curr Pharm Biotechnol. 2024. PMID: 38385486
-
HDAC4 in cancer: A multitasking platform to drive not only epigenetic modifications.Front Mol Biosci. 2023 Jan 24;10:1116660. doi: 10.3389/fmolb.2023.1116660. eCollection 2023. Front Mol Biosci. 2023. PMID: 36762207 Free PMC article. Review.
-
Stanniocalcin 2 (STC2) expression promotes post-radiation survival, migration and invasion of nasopharyngeal carcinoma cells.Cancer Manag Res. 2019 Jul 11;11:6411-6424. doi: 10.2147/CMAR.S197607. eCollection 2019. Cancer Manag Res. 2019. PMID: 31372045 Free PMC article.
-
Pro-survival Phenotype of HIF-1α: Neuroprotection Through Inflammatory Mechanisms.Adv Exp Med Biol. 2023;1438:33-36. doi: 10.1007/978-3-031-42003-0_6. Adv Exp Med Biol. 2023. PMID: 37845436 Review.
References
-
- Aebersold DM, Burri P, Beer KT, Laissue J, Djonov V, Greiner RH, Semenza GL. Expression of hypoxia-inducible factor-1alpha: a novel predictive and prognostic parameter in the radiotherapy of oropharyngeal cancer. Cancer Res. 2001;61:2911–2916. - PubMed
-
- Bazzaro M, Lee MK, Zoso A, Stirling WL, Santillan A, Shih Ie M, Roden RB. Ubiquitin-proteasome system stress sensitizes ovarian cancer to proteasome inhibitor-induced apoptosis. Cancer Res. 2006;66:3754–3763. - PubMed
-
- Benjamin D, Colombi M, Moroni C, Hall MN. Rapamycin passes the torch: a new generation of mTOR inhibitors. Nat. Rev. Drug Discov. 2011;10:868–880. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

