Estradiol regulates human QT-interval: acceleration of cardiac repolarization by enhanced KCNH2 membrane trafficking
- PMID: 26271031
- PMCID: PMC4753025
- DOI: 10.1093/eurheartj/ehv371
Estradiol regulates human QT-interval: acceleration of cardiac repolarization by enhanced KCNH2 membrane trafficking
Abstract
Background: Modulation of cardiac repolarization by sexual hormones is controversial and hormonal effects on ion channels remain largely unknown. In the present translational study, we therefore assessed the relationship between QTc duration and gonadal hormones and studied underlying mechanisms.
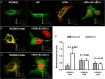
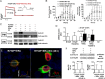
Methods and results: We measured hormone levels and QTc intervals in women during clomiphene stimulation for infertility and women before, during, and after pregnancy. Three heterozygous LQT-2 patients (KCNH2-p.Arg752Pro missense mutation) and two unaffected family members additionally were studied during their menstrual cycles. A comprehensive cellular and molecular analysis was done to identify the mechanisms of hormonal QT-interval regulation. High estradiol levels, but neither progesterone nor estradiol/progesterone ratio, inversely correlated with QTc. Consistent with clinical data, in vitro estradiol stimulation (60 pmol/L, 48 h) enhanced IKCNH2. This increase was mediated by estradiol receptor-α-dependent promotion of KCNH2-channel trafficking to the cell membrane. To study the underlying mechanism, we focused on heat-shock proteins. The heat-shock protein-90 (Hsp90) inhibitor geldanamycin abolished estradiol-induced increase in IKCNH2. Geldanamycin had no effect on KCNH2 transcription or translation; nor did it affect expression of estradiol receptors and chaperones. Estradiol enhanced the physical interaction of KCNH2-channel subunits with heat-shock proteins and augmented ion-channel trafficking to the membrane.
Conclusion: Elevated estradiol levels were associated with shorter QTc intervals in healthy women and female LQT-2 patients. Estradiol acts on KCNH2 channels via enhanced estradiol-receptor-α-mediated Hsp90 interaction, augments membrane trafficking and thereby increases repolarizing current. These results provide mechanistic insights into hormonal control of human ventricular repolarization and open novel therapeutic avenues.
Keywords: Gender; Hormones; Ion channels; Long-QT syndrome; Repolarization.
Published on behalf of the European Society of Cardiology. All rights reserved. © The Author 2015. For permissions please email: journals.permissions@oup.com.
Figures






Comment in
-
Another jigsaw piece in the complex picture of hormonal regulation of cardiac repolarization.Eur Heart J. 2016 Feb 14;37(7):651-3. doi: 10.1093/eurheartj/ehv475. Epub 2015 Sep 20. Eur Heart J. 2016. PMID: 26392434 No abstract available.
Similar articles
-
Heterogeneous Phenotype of Long QT Syndrome Caused by the KCNH2-H562R Mutation: Importance of Familial Genetic Testing.Rev Esp Cardiol (Engl Ed). 2015 Oct;68(10):861-8. doi: 10.1016/j.rec.2014.10.022. Epub 2015 Mar 26. Rev Esp Cardiol (Engl Ed). 2015. PMID: 25819988
-
KCNH2 polymorphism and methadone dosage interact to enhance QT duration.Drug Alcohol Depend. 2014 Aug 1;141:34-8. doi: 10.1016/j.drugalcdep.2014.04.027. Epub 2014 May 14. Drug Alcohol Depend. 2014. PMID: 24875677
-
Novel intracellular transport-refractory mutations in KCNH2 identified in patients with symptomatic long QT syndrome.J Cardiol. 2018 Apr;71(4):401-408. doi: 10.1016/j.jjcc.2017.10.004. Epub 2017 Nov 14. J Cardiol. 2018. PMID: 29146210
-
Influence of steroid hormones on ventricular repolarization.Pharmacol Ther. 2016 Nov;167:38-47. doi: 10.1016/j.pharmthera.2016.07.005. Epub 2016 Jul 22. Pharmacol Ther. 2016. PMID: 27452340 Review.
-
The inherited long QT syndrome: from ion channel to bedside.Cardiol Rev. 1999 Jan-Feb;7(1):44-55. Cardiol Rev. 1999. PMID: 10348966 Review.
Cited by
-
Revealing the Influences of Sex Hormones and Sex Differences in Atrial Fibrillation and Vascular Cognitive Impairment.Int J Mol Sci. 2021 Aug 16;22(16):8776. doi: 10.3390/ijms22168776. Int J Mol Sci. 2021. PMID: 34445515 Free PMC article. Review.
-
Cardiovascular profile of pharmacological agents used for the management of polycystic ovary syndrome.Ther Adv Endocrinol Metab. 2018 Nov 6;10:2042018818805674. doi: 10.1177/2042018818805674. eCollection 2019. Ther Adv Endocrinol Metab. 2018. PMID: 30800265 Free PMC article. Review.
-
Cycling matters: Sex hormone regulation of vascular potassium channels.Channels (Austin). 2023 Dec;17(1):2217637. doi: 10.1080/19336950.2023.2217637. Channels (Austin). 2023. PMID: 37243715 Free PMC article. Review.
-
Effect of long-term use of antipsychotics on the ventricular repolarization index.BMC Psychiatry. 2024 Jul 16;24(1):505. doi: 10.1186/s12888-024-05947-1. BMC Psychiatry. 2024. PMID: 39014414 Free PMC article.
-
Normalization of QT interval duration in a long QT syndrome patient during pregnancy and the postpartum period due to sex hormone effects on cardiac repolarization.HeartRhythm Case Rep. 2016 Mar 2;2(3):223-227. doi: 10.1016/j.hrcr.2015.12.012. eCollection 2016 May. HeartRhythm Case Rep. 2016. PMID: 28491674 Free PMC article. No abstract available.
References
-
- Bazett HC. An analysis of the time relationship of electrocardiograms. Heart 1920;7:353–370.
-
- Sauer AJ, Moss AJ, McNitt S, Peterson DR, Zareba W, Robinson JL, Qi M, Goldenberg I, Hobbs JB, Ackerman MJ, Benhorin J, Hall WJ, Kaufman ES, Locati EH, Napolitano C, Priori SG, Schwartz PJ, Towbin JA, Vincent GM, Zhang L. Long QT syndrome in adults. J Am Coll Cardiol 2007;49:329–337. - PubMed
-
- Ficker E, Dennis AT, Wang L, Brown AM. Role of the cytosolic chaperones Hsp70 and Hsp90 in maturation of the cardiac potassium channel HERG. Circ Res 2003;92:e87–100. - PubMed
-
- Pham TV, Rosen MR. Sex, hormones, and repolarization. Cardiovasc Res 2002;53:740–751. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

