Mycelial Mattress from a Sporangia Formation-Delayed Mutant of Rhizopus stolonifer as Wound Healing-Enhancing Biomaterial
- PMID: 26275241
- PMCID: PMC4537177
- DOI: 10.1371/journal.pone.0134090
Mycelial Mattress from a Sporangia Formation-Delayed Mutant of Rhizopus stolonifer as Wound Healing-Enhancing Biomaterial
Abstract
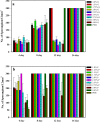
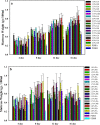
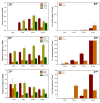
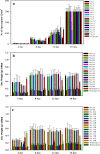
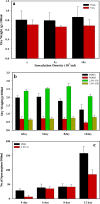
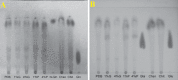
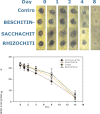
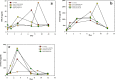
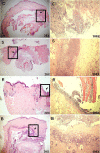
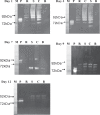
A mycelial mattress of Rhizopus stolonifer obtained from a liquid static culture was utilized for wound dressing and biomedical use. Following screening of mutants induced by UV radiation, F6, exhibiting delayed sporangium formation was selected because its sporangium maturation exhibited a 5-day delay without significant loss of mycelial weight compared to the wild type. The sporangium-free mycelial mattress from the sporangiospore culture of F6 was treated with 1N sodium hydroxide NaOH at 85°C for 2 h to produce a sponge-like membrane named Rhizochitin. The trifluoroacetic acid hydrolysate of Rhizochitin contained 36% N-acetylglucosamine and 53% hexose respectively detected by the Elson-Morgen and phenol-sulfuric acid methods. Results indicated the wound area in rats covered with Rhizochitin was 40% less than that of the uncovered group. Rhizochitin decreased the expression of PDGF in the proliferation stage, increased the expression of TGF-β in the inflammation and proliferation stages, and increased the expression of VEGF in the inflammation and proliferation stages. Rhizochitin inhibited secretion of matrix metalloproteinase-9 on days 1, 7, 9, and 12 and matrix metalloproteinase-2 on days 3, 7, 9, and 12. It was concluded that Rhizochitin has beneficial properties of biocompatible, biodegradable, and wound healing.
Conflict of interest statement
Figures


Similar articles
-
Fabrication and characterization of Rhizochitosan and its incorporation with platelet concentrates to promote wound healing.Carbohydr Polym. 2021 Sep 15;268:118239. doi: 10.1016/j.carbpol.2021.118239. Epub 2021 May 21. Carbohydr Polym. 2021. PMID: 34127221
-
Elucidation of molecular pathways responsible for the accelerated wound healing induced by a novel fibrous chitin dressing.Biomater Sci. 2019 Nov 19;7(12):5247-5257. doi: 10.1039/c9bm00404a. Biomater Sci. 2019. PMID: 31602445
-
The wound healing effects of vitamin A eye drops after a corneal alkali burn in rats.Acta Ophthalmol. 2012 Nov;90(7):e540-6. doi: 10.1111/j.1755-3768.2012.02496.x. Acta Ophthalmol. 2012. PMID: 23106861
-
Neurotensin-loaded collagen dressings reduce inflammation and improve wound healing in diabetic mice.Biochim Biophys Acta. 2014 Jan;1842(1):32-43. doi: 10.1016/j.bbadis.2013.10.009. Epub 2013 Oct 23. Biochim Biophys Acta. 2014. PMID: 24161538
-
[The modern approach to wound treatment].Med Pregl. 2000 Jul-Aug;53(7-8):363-8. Med Pregl. 2000. PMID: 11214479 Review. Croatian.
Cited by
-
From Nature to Design: Tailoring Pure Mycelial Materials for the Needs of Tomorrow.J Fungi (Basel). 2024 Feb 28;10(3):183. doi: 10.3390/jof10030183. J Fungi (Basel). 2024. PMID: 38535193 Free PMC article. Review.
-
Advanced mycelium materials as potential self-growing biomedical scaffolds.Sci Rep. 2021 Jun 16;11(1):12630. doi: 10.1038/s41598-021-91572-x. Sci Rep. 2021. PMID: 34135362 Free PMC article.
References
-
- Wu T, Zivanovic S, Draughon FA, Conway WS, Sams CE. Physicochemical properties and bioactivity of fungal chitin and chitosan. Journal of agricultural and food chemistry. 2005;53(10):3888–94. - PubMed
-
- Karimi K, Zamani A. Mucor indicus: Biology and industrial applications perspectives: A review. Biotechnology advances. 2013. - PubMed
-
- Ferreira JA, Lennartsson PR, Edebo L, Taherzadeh MJ. Zygomycetes-based biorefinery: Present status and future prospects. Bioresource technology. 2012. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

