Abnormal retinal development in Cloche mutant zebrafish
- PMID: 26283463
- PMCID: PMC4619121
- DOI: 10.1002/dvdy.24322
Abnormal retinal development in Cloche mutant zebrafish
Abstract
Background: Functions for the early embryonic vasculature in regulating development of central nervous system tissues, such as the retina, have been suggested by in vitro studies and by in vivo manipulations that caused additional ocular vessels to develop. Here, we use an avascular zebrafish embryo, cloche-/- (clo-/-), to begin to identify necessary developmental functions of the ocular vasculature in regulating development and patterning of the neural retina, in vivo. These studies are possible in zebrafish embryos, which do not yet rely upon the vasculature for tissue oxygenation.
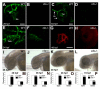
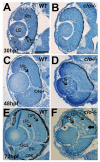
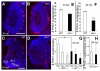
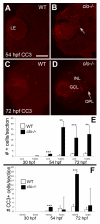
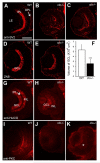
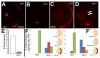
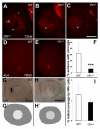
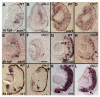
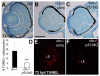
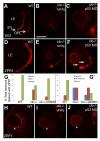
Results: clo-/- embryos lacked early ocular vasculature and were microphthalmic, with reduced retinal cell proliferation and cell survival. Retinas of clo mutants were disorganized, with irregular synaptic layers, mispatterned expression domains of retinal transcription factors, morphologically abnormal Müller glia, reduced differentiation of specific retinal cell types, and sporadically distributed cone photoreceptors. Blockade of p53-mediated cell death did not completely rescue this phenotype and revealed ectopic cones in the inner nuclear layer. clo-/- embryos did not upregulate a molecular marker for hypoxia.
Conclusions: The disorganized retinal phenotype of clo-/- embryos is consistent with a neural and glial developmental patterning role for the early ocular vasculature that is independent of its eventual function in gas exchange.
Keywords: Müller glia; neurod1; neurogenesis; pax6a; photoreceptors; retina; vasculature; zebrafish.
© 2015 Wiley Periodicals, Inc.
Figures

Similar articles
-
dazed gene is necessary for late cell type development and retinal cell maintenance in the zebrafish retina.Dev Dyn. 2005 Jun;233(2):680-94. doi: 10.1002/dvdy.20375. Dev Dyn. 2005. PMID: 15844196
-
Expanded progenitor populations, vitreo-retinal abnormalities, and Müller glial reactivity in the zebrafish leprechaun/patched2 retina.BMC Dev Biol. 2009 Oct 19;9:52. doi: 10.1186/1471-213X-9-52. BMC Dev Biol. 2009. PMID: 19840373 Free PMC article.
-
Sox2 regulates Müller glia reprogramming and proliferation in the regenerating zebrafish retina via Lin28 and Ascl1a.Exp Eye Res. 2017 Aug;161:174-192. doi: 10.1016/j.exer.2017.05.012. Epub 2017 May 31. Exp Eye Res. 2017. PMID: 28577895 Free PMC article.
-
The Use of Stem Cell Differentiation Stage Factors (SCDSFs) Taken from Zebrafish Embryos during Organogenesis and Their Role in Regulating the Gene Expression of Normal and Pathological (Stem) Cells.Int J Mol Sci. 2020 Jul 12;21(14):4914. doi: 10.3390/ijms21144914. Int J Mol Sci. 2020. PMID: 32664640 Free PMC article. Review.
-
Harnessing the power of forward genetics--analysis of neuronal diversity and patterning in the zebrafish retina.Trends Neurosci. 2000 Nov;23(11):531-41. doi: 10.1016/s0166-2236(00)01655-6. Trends Neurosci. 2000. PMID: 11074262 Review.
Cited by
-
Concerted regulation of retinal pigment epithelium basement membrane and barrier function by angiocrine factors.Nat Commun. 2017 May 19;8:15374. doi: 10.1038/ncomms15374. Nat Commun. 2017. PMID: 28524846 Free PMC article.
-
Phenotype-based Discovery of 2-[(E)-2-(Quinolin-2-yl)vinyl]phenol as a Novel Regulator of Ocular Angiogenesis.J Biol Chem. 2016 Apr 1;291(14):7242-55. doi: 10.1074/jbc.M115.710665. Epub 2016 Feb 4. J Biol Chem. 2016. PMID: 26846851 Free PMC article.
-
Zebrafish models of alx-linked frontonasal dysplasia reveal a role for Alx1 and Alx3 in the anterior segment and vasculature of the developing eye.Biol Open. 2022 May 15;11(5):bio059189. doi: 10.1242/bio.059189. Epub 2022 Jun 7. Biol Open. 2022. PMID: 35142342 Free PMC article.
-
Vascular Regulation of Developmental Neurogenesis.Front Cell Dev Biol. 2022 Apr 29;10:890852. doi: 10.3389/fcell.2022.890852. eCollection 2022. Front Cell Dev Biol. 2022. PMID: 35573692 Free PMC article. Review.
-
Why does the zebrafish cloche mutant develop lens cataract?PLoS One. 2019 Mar 12;14(3):e0211399. doi: 10.1371/journal.pone.0211399. eCollection 2019. PLoS One. 2019. PMID: 30861003 Free PMC article.
References
-
- Aizawa Y, Shoichet MS. The role of endothelial cells in the retinal stem and progenitor cell niche within a 3D engineered hydrogel matrix. Biomaterials. 2012;33:5198–5205. - PubMed
-
- Barthel LK, Raymond PA. Improved method for obtaining 3-microns cryosections for immunocytochemistry. The journal of histochemistry and cytochemistry : official journal of the Histochemistry Society. 1990;38:1383–1388. - PubMed
-
- Chen S, Wang QL, Nie Z, Sun H, Lennon G, Copeland NG, Gilbert DJ, Jenkins NA, Zack DJ. Crx, a novel Otx-like paired-homeodomain protein, binds to and transactivates photoreceptor cell-specific genes. Neuron. 1997;19:1017–1030. - PubMed
-
- Chuang JC, Mathers PH, Raymond PA. Expression of three Rx homeobox genes in embryonic and adult zebrafish. Mechanisms of development. 1999;84:195–198. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

