Fluoride induces oxidative damage and SIRT1/autophagy through ROS-mediated JNK signaling
- PMID: 26431905
- PMCID: PMC4684823
- DOI: 10.1016/j.freeradbiomed.2015.08.015
Fluoride induces oxidative damage and SIRT1/autophagy through ROS-mediated JNK signaling
Abstract
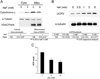
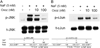
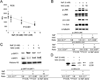
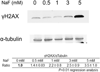
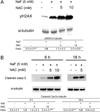
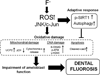
Fluoride is an effective caries prophylactic, but at high doses can also be an environmental health hazard. Acute or chronic exposure to high fluoride doses can result in dental enamel and skeletal and soft tissue fluorosis. Dental fluorosis is manifested as mottled, discolored, porous enamel that is susceptible to dental caries. Fluoride induces cell stress, including endoplasmic reticulum stress and oxidative stress, which leads to impairment of ameloblasts responsible for dental enamel formation. Recently we reported that fluoride activates SIRT1 and autophagy as an adaptive response to protect cells from stress. However, it still remains unclear how SIRT1/autophagy is regulated in dental fluorosis. In this study, we demonstrate that fluoride exposure generates reactive oxygen species (ROS) and the resulting oxidative damage is counteracted by SIRT1/autophagy induction through c-Jun N-terminal kinase (JNK) signaling in ameloblasts. In the mouse-ameloblast-derived cell line LS8, fluoride induced ROS, mitochondrial damage including cytochrome-c release, up-regulation of UCP2, attenuation of ATP synthesis, and H2AX phosphorylation (γH2AX), which is a marker of DNA damage. We evaluated the effects of the ROS inhibitor N-acetylcysteine (NAC) and the JNK inhibitor SP600125 on fluoride-induced SIRT1/autophagy activation. NAC decreased fluoride-induced ROS generation and attenuated JNK and c-Jun phosphorylation. NAC decreased SIRT1 phosphorylation and formation of the autophagy marker LC3II, which resulted in an increase in the apoptosis mediators γH2AX and cleaved/activated caspase-3. SP600125 attenuated fluoride-induced SIRT1 phosphorylation, indicating that fluoride activates SIRT1/autophagy via the ROS-mediated JNK pathway. In enamel organs from rats or mice treated with 50, 100, or 125 ppm fluoride for 6 weeks, cytochrome-c release and the DNA damage markers 8-oxoguanine, p-ATM, and γH2AX were increased compared to those in controls (0 ppm fluoride). These results suggest that fluoride-induced ROS generation causes mitochondrial damage and DNA damage, which may lead to impairment of ameloblast function. To counteract this impairment, SIRT1/autophagy is induced via JNK signaling to protect cells/ameloblasts from fluoride-induced oxidative damage that may cause dental fluorosis.
Keywords: Ameloblast; Autophagy; Fluorosis; JNK; Oxidative damage; ROS; Sirtuin.
Copyright © 2015 Elsevier Inc. All rights reserved.
Conflict of interest statement
None.
Figures



Similar articles
-
Sirtuin1 and autophagy protect cells from fluoride-induced cell stress.Biochim Biophys Acta. 2014 Feb;1842(2):245-55. doi: 10.1016/j.bbadis.2013.11.023. Epub 2013 Dec 1. Biochim Biophys Acta. 2014. PMID: 24296261 Free PMC article.
-
Sirt1 overexpression suppresses fluoride-induced p53 acetylation to alleviate fluoride toxicity in ameloblasts responsible for enamel formation.Arch Toxicol. 2018 Mar;92(3):1283-1293. doi: 10.1007/s00204-017-2135-2. Epub 2017 Nov 28. Arch Toxicol. 2018. PMID: 29185024 Free PMC article.
-
Vitamin E and lycopene reduce coal burning fluorosis-induced spermatogenic cell apoptosis via oxidative stress-mediated JNK and ERK signaling pathways.Biosci Rep. 2018 Jul 31;38(4):BSR20171003. doi: 10.1042/BSR20171003. Print 2018 Aug 31. Biosci Rep. 2018. PMID: 29273675 Free PMC article.
-
P66Shc-SIRT1 Regulation of Oxidative Stress Protects Against Cardio-cerebral Vascular Disease.Mol Neurobiol. 2017 Sep;54(7):5277-5285. doi: 10.1007/s12035-016-0073-2. Epub 2016 Aug 30. Mol Neurobiol. 2017. PMID: 27578018 Review.
-
Regulation of the effects of CYP2E1-induced oxidative stress by JNK signaling.Redox Biol. 2014;3:7-15. doi: 10.1016/j.redox.2014.09.004. Epub 2014 Sep 23. Redox Biol. 2014. PMID: 25462060 Free PMC article. Review.
Cited by
-
Sodium fluoride exposure exerts toxic effects on porcine oocyte maturation.Sci Rep. 2017 Dec 6;7(1):17082. doi: 10.1038/s41598-017-17357-3. Sci Rep. 2017. PMID: 29213094 Free PMC article.
-
4-phenylbutyrate Mitigates Fluoride-Induced Cytotoxicity in ALC Cells.Front Physiol. 2017 May 11;8:302. doi: 10.3389/fphys.2017.00302. eCollection 2017. Front Physiol. 2017. PMID: 28553235 Free PMC article.
-
Role of Mitogen Activated Protein Kinase Signaling in Parkinson's Disease.Int J Mol Sci. 2018 Sep 29;19(10):2973. doi: 10.3390/ijms19102973. Int J Mol Sci. 2018. PMID: 30274251 Free PMC article. Review.
-
Long-term exposure to low level of fluoride induces apoptosis via p53 pathway in lymphocytes of aluminum smelter workers.Environ Sci Pollut Res Int. 2019 Jan;26(3):2671-2680. doi: 10.1007/s11356-018-3726-z. Epub 2018 Nov 26. Environ Sci Pollut Res Int. 2019. PMID: 30478774
-
Biological Activities and Potential Oral Applications of N-Acetylcysteine: Progress and Prospects.Oxid Med Cell Longev. 2018 Apr 22;2018:2835787. doi: 10.1155/2018/2835787. eCollection 2018. Oxid Med Cell Longev. 2018. PMID: 29849877 Free PMC article. Review.
References
-
- CDC. Recommendations for using fluoride to prevent and control dental caries in the United States. Centers for Disease Control and Prevention. MMWR. Recommendations and reports: Morbidity and mortality weekly report. Recommendations and reports/Centers for Disease Control. 2001;50:1–42. - PubMed
-
- Boivin G, Chavassieux P, Chapuy MC, Baud CA, Meunier PJ. Skeletal fluorosis: histomorphometric analysis of bone changes and bone fluoride content in 29 patients. Bone. 1989;10:89–99. - PubMed
-
- Thrane EV, Refsnes M, Thoresen GH, Lag M, Schwarze PE. Fluoride-induced apoptosis in epithelial lung cells involves activation of MAP kinases p38 and possibly JNK. Toxicological sciences: an official journal of the Society of Toxicology. 2001;61:83–91. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

