Early Growth Response 1 (Egr-1) Regulates N-Methyl-d-aspartate Receptor (NMDAR)-dependent Transcription of PSD-95 and α-Amino-3-hydroxy-5-methyl-4-isoxazole Propionic Acid Receptor (AMPAR) Trafficking in Hippocampal Primary Neurons
- PMID: 26475861
- PMCID: PMC4705959
- DOI: 10.1074/jbc.M115.668889
Early Growth Response 1 (Egr-1) Regulates N-Methyl-d-aspartate Receptor (NMDAR)-dependent Transcription of PSD-95 and α-Amino-3-hydroxy-5-methyl-4-isoxazole Propionic Acid Receptor (AMPAR) Trafficking in Hippocampal Primary Neurons
Abstract
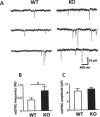
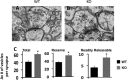
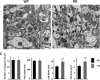
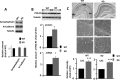
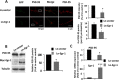
The N-methyl-d-aspartate receptor (NMDAR) controls synaptic plasticity and memory function and is one of the major inducers of transcription factor Egr-1 in the hippocampus. However, how Egr-1 mediates the NMDAR signal in neurons has remained unclear. Here, we show that the hippocampus of mice lacking Egr-1 displays electrophysiology properties and ultrastructure that are similar to mice overexpressing PSD-95, a major scaffolding protein of postsynaptic density involved in synapse formation, synaptic plasticity, and synaptic _targeting of AMPA receptors (AMPARs), which mediate the vast majority of excitatory transmission in the CNS. We demonstrate that Egr-1 is a transcription repressor of the PSD-95 gene and is recruited to the PSD-95 promoter in response to NMDAR activation. Knockdown of Egr-1 in rat hippocampal primary neurons blocks NMDAR-induced PSD-95 down-regulation and AMPAR endocytosis. Likewise, overexpression of Egr-1 in rat hippocampal primary neurons causes reduction in PSD-95 protein level and promotes AMPAR endocytosis. Our data indicate that Egr-1 is involved in NMDAR-mediated PSD-95 down-regulation and AMPAR endocytosis, a process important in the expression of long term depression.
Keywords: PSD95; early growth response protein 1 (EGR1); endocytosis; neurobiology; transcription regulation; α-amino-3-hydroxy-5-methyl-4-isoxazole propionic acid receptor (AMPA receptor, AMPAR).
© 2015 by The American Society for Biochemistry and Molecular Biology, Inc.
Figures





Similar articles
-
A critical role for PSD-95/AKAP interactions in endocytosis of synaptic AMPA receptors.Nat Neurosci. 2009 Feb;12(2):172-81. doi: 10.1038/nn.2249. Epub 2009 Jan 25. Nat Neurosci. 2009. PMID: 19169250 Free PMC article.
-
BRAG2a Mediates mGluR-Dependent AMPA Receptor Internalization at Excitatory Postsynapses through the Interaction with PSD-95 and Endophilin 3.J Neurosci. 2020 May 27;40(22):4277-4296. doi: 10.1523/JNEUROSCI.1645-19.2020. Epub 2020 Apr 27. J Neurosci. 2020. PMID: 32341099 Free PMC article.
-
Differential requirement for NMDAR activity in SAP97β-mediated regulation of the number and strength of glutamatergic AMPAR-containing synapses.J Neurophysiol. 2014 Feb;111(3):648-58. doi: 10.1152/jn.00262.2013. Epub 2013 Nov 13. J Neurophysiol. 2014. PMID: 24225540 Free PMC article.
-
Regulation of neuronal PKA signaling through AKAP _targeting dynamics.Eur J Cell Biol. 2006 Jul;85(7):627-33. doi: 10.1016/j.ejcb.2006.01.010. Epub 2006 Feb 28. Eur J Cell Biol. 2006. PMID: 16504338 Review.
-
Methods for Uncovering the Mechanisms of AMPA Receptor Trafficking.In: Kittler JT, Moss SJ, editors. The Dynamic Synapse: Molecular Methods in Ionotropic Receptor Biology. Boca Raton (FL): CRC Press/Taylor & Francis; 2006. Chapter 1. In: Kittler JT, Moss SJ, editors. The Dynamic Synapse: Molecular Methods in Ionotropic Receptor Biology. Boca Raton (FL): CRC Press/Taylor & Francis; 2006. Chapter 1. PMID: 21204482 Free Books & Documents. Review.
Cited by
-
CircHTT(2,3,4,5,6) - co-evolving with the HTT CAG-repeat tract - modulates Huntington's disease phenotypes.Mol Ther Nucleic Acids. 2024 Jun 3;35(3):102234. doi: 10.1016/j.omtn.2024.102234. eCollection 2024 Sep 10. Mol Ther Nucleic Acids. 2024. PMID: 38974999 Free PMC article.
-
Changes in GABA and glutamate receptors on auditory cortical excitatory neurons in a rat model of salicylate-induced tinnitus.Am J Transl Res. 2018 Dec 15;10(12):3941-3955. eCollection 2018. Am J Transl Res. 2018. PMID: 30662641 Free PMC article.
-
The Role of Early Growth Response 1 (EGR1) in Brain Plasticity and Neuropsychiatric Disorders.Front Behav Neurosci. 2017 Mar 6;11:35. doi: 10.3389/fnbeh.2017.00035. eCollection 2017. Front Behav Neurosci. 2017. PMID: 28321184 Free PMC article. Review.
-
Early Growth Response 1 (Egr-1) Is a Transcriptional Activator of β-Secretase 1 (BACE-1) in the Brain.J Biol Chem. 2016 Oct 14;291(42):22276-22287. doi: 10.1074/jbc.M116.738849. Epub 2016 Aug 30. J Biol Chem. 2016. PMID: 27576688 Free PMC article.
-
Evidence that CA3 is Underling the Comorbidity Between Pain and Depression and the Co-curation by Wu-Tou decoction in Neuropathic Pain.Sci Rep. 2017 Sep 20;7(1):11935. doi: 10.1038/s41598-017-12184-y. Sci Rep. 2017. PMID: 28931876 Free PMC article.
References
-
- Alvarez V. A., and Sabatini B. L. (2007) Anatomical and physiological plasticity of dendritic spines. Annu. Rev. Neurosci. 30, 79–97 - PubMed
-
- Lüscher C., Nicoll R. A., Malenka R. C., and Muller D. (2000) Synaptic plasticity and dynamic modulation of the postsynaptic membrane. Nat. Neurosci. 3, 545–550 - PubMed
-
- Kennedy M. B. (2000) Signal-processing machines at the postsynaptic density. Science 290, 750–754 - PubMed
-
- Sheng M., and Kim M. J. (2002) Postsynaptic signaling and plasticity mechanisms. Science 298, 776–780 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials

