Diurnal Glycemic Patterns during an 8-Week Open-Label Proof-of-Concept Trial of Empagliflozin in Type 1 Diabetes
- PMID: 26544192
- PMCID: PMC4636141
- DOI: 10.1371/journal.pone.0141085
Diurnal Glycemic Patterns during an 8-Week Open-Label Proof-of-Concept Trial of Empagliflozin in Type 1 Diabetes
Abstract
Background: We recently reported improved glycemic control with reduced insulin dose in subjects with type 1 diabetes treated with the sodium glucose co-transporter-2 inhibitor empagliflozin. To further characterize the effects, we analyzed diurnal glycemic patterns by continuous glucose monitoring (CGM).
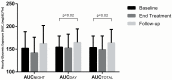
Methods: In an 8-week single-arm open-label pilot study of empagliflozin, we compared ambulatory glucose profiles produced from CGM data during 2-week intervals in a placebo run-in baseline period, end-of-treatment, and post-treatment. Change in glycemic exposure was evaluated by area under the median curve according to time of day (AUCTOTAL 12:00am-11:55pm; AUCDAY 7:05am-10:55pm, AUCNIGHT 11:00pm-7:00am), as well as glycemic variability, glycemic stability and time-in-_target (≥70 to ≤140mg/dL).
Results: The 40 patients (26 on insulin pump) were aged 24±5 years and BMI 24.5±3.2 kg/m2. Consistent with the observed HbA1c decrease (8.0±0.9% to 7.6±0.9%, p<0.0001), normalized AUCTOTAL CGM decreased from 153.7±25.4 to 149.0±30.2mg/dL∙h at end-of-treatment (p = 0.31), and significantly increased post-treatment (164.1±29.5mg/dL∙h, p = 0.02). The numerical decrease in normalized AUCNIGHT (152.0±36.6 to 141.9±34.4mg/dL∙h, p = 0.13) exceeded AUCDAY (154.5±24.5 to 152.6±30.4mg/dL∙h, p = 0.65). Trends toward lower glycemic variability (83.1±18.9 to 75.6±28.6mg/dL, p = 0.06) and little change in glycemic stability (10.8±3.6 to 10.3±4.5mg/dL/h, p = 0.51) were observed. When empagliflozin was discontinued, these worsened relative to baseline (89.3±19.3mg/dL, p = 0.04 and 11.8±3.7mg/dL/hr, p = 0.08). Time-in-_target numerically increased (40.2±11.9 to 43.1±13.5%, p = 0.69) at end-of-treatment but reversed post-treatment. Findings were similar on stratification of pump and MDI subjects.
Conclusions: We observed that empagliflozin was associated with patterns of improved nighttime glycemia more prominent than daytime.
Trial registration: Clinicaltrials.gov NCT01392560.
Conflict of interest statement
Figures
Similar articles
-
Glucose Exposure and Variability with Empagliflozin as Adjunct to Insulin in Patients with Type 1 Diabetes: Continuous Glucose Monitoring Data from a 4-Week, Randomized, Placebo-Controlled Trial (EASE-1).Diabetes Technol Ther. 2017 Jan;19(1):49-60. doi: 10.1089/dia.2016.0261. Epub 2016 Dec 8. Diabetes Technol Ther. 2017. PMID: 27929674 Clinical Trial.
-
Sodium-glucose cotransporter 2 inhibition and glycemic control in type 1 diabetes: results of an 8-week open-label proof-of-concept trial.Diabetes Care. 2014 May;37(5):1480-3. doi: 10.2337/dc13-2338. Epub 2014 Mar 4. Diabetes Care. 2014. PMID: 24595630 Clinical Trial.
-
Effect of empagliflozin monotherapy on postprandial glucose and 24-hour glucose variability in Japanese patients with type 2 diabetes mellitus: a randomized, double-blind, placebo-controlled, 4-week study.Cardiovasc Diabetol. 2015 Jan 30;14:11. doi: 10.1186/s12933-014-0169-9. Cardiovasc Diabetol. 2015. PMID: 25633683 Free PMC article. Clinical Trial.
-
8. Pharmacologic Approaches to Glycemic Treatment.Diabetes Care. 2017 Jan;40(Suppl 1):S64-S74. doi: 10.2337/dc17-S011. Diabetes Care. 2017. PMID: 27979895 Review. No abstract available.
-
7. Approaches to Glycemic Treatment.Diabetes Care. 2016 Jan;39 Suppl 1:S52-9. doi: 10.2337/dc16-S010. Diabetes Care. 2016. PMID: 26696682 Review. No abstract available.
Cited by
-
The Impact of Sotagliflozin on Renal Function, Albuminuria, Blood Pressure, and Hematocrit in Adults With Type 1 Diabetes.Diabetes Care. 2019 Oct;42(10):1921-1929. doi: 10.2337/dc19-0937. Epub 2019 Aug 1. Diabetes Care. 2019. PMID: 31371432 Free PMC article. Clinical Trial.
-
Adoption of New Glucose-Lowering Medications in the U.S.-The Case of SGLT2 Inhibitors: Nationwide Cohort Study.Diabetes Technol Ther. 2019 Dec;21(12):702-712. doi: 10.1089/dia.2019.0213. Epub 2019 Oct 9. Diabetes Technol Ther. 2019. PMID: 31418588 Free PMC article.
-
Empagliflozin in type 1 diabetes.Diabetes Metab Syndr Obes. 2019 Aug 22;12:1555-1561. doi: 10.2147/DMSO.S194688. eCollection 2019. Diabetes Metab Syndr Obes. 2019. PMID: 31686876 Free PMC article. Review.
-
The efficacy and safety of SGLT2 inhibitors for adjunctive treatment of type 1 diabetes: a systematic review and meta-analysis.Sci Rep. 2017 Mar 9;7:44128. doi: 10.1038/srep44128. Sci Rep. 2017. PMID: 28276512 Free PMC article. Review.
-
Canagliflozin in Type 1 Diabetes: A Case Series of Patient Outcomes in a Diabetes Clinic.Diabetes Spectr. 2019 Feb;32(1):47-51. doi: 10.2337/ds17-0018. Diabetes Spectr. 2019. PMID: 30853764 Free PMC article. No abstract available.
References
-
- Imran SA, Rabasa-Lhoret R, Ross S, Canadian Diabetes Association Clinical Practice Guidelines Expert Committee. _targets for glycemic control. Can J Diabetes. 2013;37 Suppl 1:S31–4. - PubMed
-
- Wood J, Miller K, Maahs D, Beck R, Dimeglio L, Libman I, et al. T1D Exchange clinic network. Most youth with type 1 diabetes in the T1D exchange clinic registry do not meet American Diabetes Association or Internation Society for Pediatric and Adolescent Diabetes Clinical Guidelines. Diabetes Care. 2013;36:2035–7. 10.2337/dc12-1959 - DOI - PMC - PubMed
-
- Gerstl EM, Rabl W, Resenbauer J, Grobe H, Hofer SE, Krause U, et al. Metabolic control as reflected by HbA1c in children, adolescents and young adults with type-1 diabetes mellitus: combined longitudinal analysis including 27,035 patients from 207 centers in Germany and Austria during the last decade. Eur J Pediatr. 2008;167(4):447–53. - PubMed
-
- The Diabetes Control and Compications Research Group. Weight gain associated with intensive therapy in the diabetes control and complication trial. Diabetes Care. 1988;11(7):567–73. - PubMed
-
- The Diabetes Control and Compications Research Group. The effect of intensive treatment of diabetes on the development and progression of long-term complications in insulin-dependent diabetes mellitus. N Engl J Med. 1993;329(14):977–86. - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

