Ordinary and Activated Bone Grafts: Applied Classification and the Main Features
- PMID: 26649300
- PMCID: PMC4662978
- DOI: 10.1155/2015/365050
Ordinary and Activated Bone Grafts: Applied Classification and the Main Features
Abstract
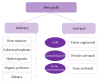
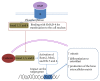
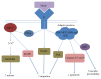
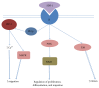
Bone grafts are medical devices that are in high demand in clinical practice for substitution of bone defects and recovery of atrophic bone regions. Based on the analysis of the modern groups of bone grafts, the particularities of their composition, the mechanisms of their biological effects, and their therapeutic indications, applicable classification was proposed that separates the bone substitutes into "ordinary" and "activated." The main differential criterion is the presence of biologically active components in the material that are standardized by qualitative and quantitative parameters: growth factors, cells, or gene constructions encoding growth factors. The pronounced osteoinductive and (or) osteogenic properties of activated osteoplastic materials allow drawing upon their efficacy in the substitution of large bone defects.
Figures
Similar articles
-
The use of bone graft substitutes in large cancellous voids: any specific needs?Injury. 2011 Sep;42 Suppl 2:S87-90. doi: 10.1016/j.injury.2011.06.020. Epub 2011 Jul 2. Injury. 2011. PMID: 21723553
-
[Bone substitutes: Classification and concerns].Rev Stomatol Chir Maxillofac. 2011 Sep;112(4):212-21. doi: 10.1016/j.stomax.2011.06.003. Epub 2011 Jul 23. Rev Stomatol Chir Maxillofac. 2011. PMID: 21783214 French.
-
Bone graft substitutes: What are the options?Surgeon. 2012 Aug;10(4):230-9. doi: 10.1016/j.surge.2012.04.001. Epub 2012 Jun 6. Surgeon. 2012. Retraction in: Surgeon. 2013 Apr;11(2):115. PMID: 22682580 Retracted. Review.
-
Bone substitutes: an update.Injury. 2005 Nov;36 Suppl 3:S20-7. doi: 10.1016/j.injury.2005.07.029. Injury. 2005. PMID: 16188545 Review.
-
Osseous grafting part II: xenografts and alloplasts for periodontal regeneration--a literature review.J Int Acad Periodontol. 2010 Apr;12(2):39-44. J Int Acad Periodontol. 2010. PMID: 20465030 Review.
Cited by
-
Bone Augmentation for Implant Placement: Recent Advances.Int J Dent. 2022 Mar 27;2022:8900940. doi: 10.1155/2022/8900940. eCollection 2022. Int J Dent. 2022. PMID: 35386549 Free PMC article. Review.
-
Comment on "World's First Clinical Case of Gene-Activated Bone Substitute Application".Case Rep Dent. 2017;2017:4383926. doi: 10.1155/2017/4383926. Epub 2017 Jul 12. Case Rep Dent. 2017. PMID: 28785490 Free PMC article. No abstract available.
-
Endogenous Regeneration of Alveolar Bone by Decellularized Tooth Matrix.Bull Exp Biol Med. 2023 Aug;175(4):592-599. doi: 10.1007/s10517-023-05908-w. Epub 2023 Sep 28. Bull Exp Biol Med. 2023. PMID: 37768453
-
Injectable porcine bone demineralized and digested extracellular matrix-PEGDA hydrogel blend for bone regeneration.J Mater Sci Mater Med. 2020 Jan 27;31(2):21. doi: 10.1007/s10856-019-6354-3. J Mater Sci Mater Med. 2020. PMID: 31989310
-
Physical/Chemical Properties and Resorption Behavior of a Newly Developed Ca/P/S-Based Bone Substitute Material.Materials (Basel). 2020 Aug 5;13(16):3458. doi: 10.3390/ma13163458. Materials (Basel). 2020. PMID: 32764505 Free PMC article.
References
-
- Number of all-listed procedures for discharges from shortly-stay hospitals, 2010, http://www.cdc.gov/nchs/data/nhds/10Detaileddiagnosesprocedures/2010det1....
-
- Chiapasco M., Casentini P., Zaniboni M. Bone augmentation procedures in implant dentistry. The International Journal of Oral & Maxillofacial Implants. 2009;24:237–259. - PubMed
-
- Annual report 2013, Turning a new page, Straumann, http://www.straumann.com/content/dam/internet/straumann_com/Resources/in....
-
- Deev R. V., Bozo I. Y. Evolution of Bone Grafts, Materials of the V Russian Symposium with International Participation. Ufa, Russia: Bashkortostan Publishing; 2012. (edited by: E. R. Muldashev).
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

