Neuroinflammation and Depression: Microglia Activation, Extracellular Microvesicles and microRNA Dysregulation
- PMID: 26733805
- PMCID: PMC4681811
- DOI: 10.3389/fncel.2015.00476
Neuroinflammation and Depression: Microglia Activation, Extracellular Microvesicles and microRNA Dysregulation
Abstract
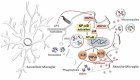
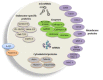
Patients with chronic inflammation are often associated with the emergence of depression symptoms, while diagnosed depressed patients show increased levels of circulating cytokines. Further studies revealed the activation of the brain immune cell microglia in depressed patients with a greater magnitude in individuals that committed suicide, indicating a crucial role for neuroinflammation in depression brain pathogenesis. Rapid advances in the understanding of microglial and astrocytic neurobiology were obtained in the past 15-20 years. Indeed, recent data reveal that microglia play an important role in managing neuronal cell death, neurogenesis, and synaptic interactions, besides their involvement in immune-response generating cytokines. The communication between microglia and neurons is essential to synchronize these diverse functions with brain activity. Evidence is accumulating that secreted extracellular vesicles (EVs), comprising ectosomes and exosomes with a size ranging from 0.1-1 μm, are key players in intercellular signaling. These EVs may carry specific proteins, mRNAs and microRNAs (miRNAs). Transfer of exosomes to neurons was shown to be mediated by oligodendrocytes, microglia and astrocytes that may either be supportive to neurons, or instead disseminate the disease. Interestingly, several recent reports have identified changes in miRNAs in depressed patients, which _target not only crucial pathways associated with synaptic plasticity, learning and memory but also the production of neurotrophic factors and immune cell modulation. In this article, we discuss the role of neuroinflammation in the emergence of depression, namely dynamic alterations in the status of microglia response to stimulation, and how their activation phenotypes may have an etiological role in neurodegeneneration, in particular in depressive-like behavior. We will overview the involvement of miRNAs, exosomes, ectosomes and microglia in regulating critical pathways associated with depression and how they may contribute to other brain disorders including amyotrophic lateral sclerosis (ALS), Alzheimer's disease (AD) and Parkinson's disease (PD), which share several neuroinflammatory-associated processes. Specific reference will be made to EVs as potential biomarkers and disease monitoring approaches, focusing on their potentialities as drug delivery vehicles, and on putative therapeutic strategies using autologous exosome-based delivery systems to treat neurodegenerative and psychiatric disorders.
Keywords: astrocytes; ectosomes; exosomes; glia interplay; microRNAs; microglia; neurodegeneration; oligodendrocytes.
Figures
Similar articles
-
Microglial Exosomes in Neurodegenerative Disease.Front Mol Neurosci. 2021 May 11;14:630808. doi: 10.3389/fnmol.2021.630808. eCollection 2021. Front Mol Neurosci. 2021. PMID: 34045943 Free PMC article. Review.
-
Brain incoming call from glia during neuroinflammation: Roles of extracellular vesicles.Neurobiol Dis. 2024 Oct 15;201:106663. doi: 10.1016/j.nbd.2024.106663. Epub 2024 Sep 7. Neurobiol Dis. 2024. PMID: 39251030 Review.
-
Microglia-derived extracellular vesicles in Alzheimer's Disease: A double-edged sword.Biochem Pharmacol. 2018 Feb;148:184-192. doi: 10.1016/j.bcp.2017.12.020. Epub 2018 Jan 3. Biochem Pharmacol. 2018. PMID: 29305855 Review.
-
Astrocyte-derived extracellular vesicles: Neuroreparative properties and role in the pathogenesis of neurodegenerative disorders.J Control Release. 2020 Jul 10;323:225-239. doi: 10.1016/j.jconrel.2020.04.017. Epub 2020 Apr 11. J Control Release. 2020. PMID: 32289328 Free PMC article. Review.
-
Extracellular vesicles as mediators of neuron-glia communication.Front Cell Neurosci. 2013 Oct 30;7:182. doi: 10.3389/fncel.2013.00182. Front Cell Neurosci. 2013. PMID: 24194697 Free PMC article. Review.
Cited by
-
Chronic interleukin-6 mediated neuroinflammation decreases anxiety, and impaires spatial memory in aged female mice.Front Neurosci. 2023 Nov 23;17:1267818. doi: 10.3389/fnins.2023.1267818. eCollection 2023. Front Neurosci. 2023. PMID: 38075266 Free PMC article.
-
The natural defense system and the normative self model.F1000Res. 2016 May 3;5:797. doi: 10.12688/f1000research.8518.1. eCollection 2016. F1000Res. 2016. PMID: 27303629 Free PMC article.
-
A Reflection Upon the Contribution of Retinal and Cortical Electrophysiology to Time of Information Processing in Psychiatric Disorders.Front Psychiatry. 2022 Apr 5;13:856498. doi: 10.3389/fpsyt.2022.856498. eCollection 2022. Front Psychiatry. 2022. PMID: 35449563 Free PMC article. No abstract available.
-
Extracellular Vesicles in Mental Disorders: A State-of-art Review.Int J Biol Sci. 2023 Feb 5;19(4):1094-1109. doi: 10.7150/ijbs.79666. eCollection 2023. Int J Biol Sci. 2023. PMID: 36923936 Free PMC article. Review.
-
Cochlear resident macrophage mediates development of ribbon synapses via CX3CR1/CX3CL1 axis.Front Mol Neurosci. 2022 Nov 28;15:1031278. doi: 10.3389/fnmol.2022.1031278. eCollection 2022. Front Mol Neurosci. 2022. PMID: 36518186 Free PMC article.
References
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

