Adapted physical exercise enhances activation and differentiation potential of satellite cells in the skeletal muscle of old mice
- PMID: 26739770
- PMCID: PMC4831340
- DOI: 10.1111/joa.12429
Adapted physical exercise enhances activation and differentiation potential of satellite cells in the skeletal muscle of old mice
Abstract
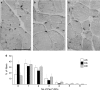
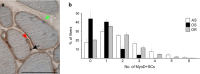
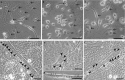
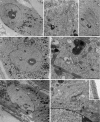
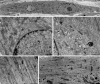
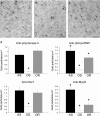
During ageing, a progressive loss of skeletal muscle mass and a decrease in muscle strength and endurance take place, in the condition termed sarcopenia. The mechanisms of sarcopenia are complex and still unclear; however, it is known that muscle atrophy is associated with a decline in the number and/or efficiency of satellite cells, the main contributors to muscle regeneration. Physical exercise proved beneficial in sarcopenia; however, knowledge of the effect of adapted physical exercise on the myogenic properties of satellite cells in aged muscles is limited. In this study the amount and activation state of satellite cells as well as their proliferation and differentiation potential were assessed in situ by morphology, morphometry and immunocytochemistry at light and transmission electron microscopy on 28-month-old mice submitted to adapted aerobic physical exercise on a treadmill. Sedentary age-matched mice served as controls, and sedentary adult mice were used as a reference for an unperturbed control at an age when the capability of muscle regeneration is still high. The effect of physical exercise in aged muscles was further analysed by comparing the myogenic potential of satellite cells isolated from old running and old sedentary mice using an in vitro system that allows observation of the differentiation process under controlled experimental conditions. The results of this ex vivo and in vitro study demonstrated that adapted physical exercise increases the number and activation of satellite cells as well as their capability to differentiate into structurally and functionally correct myotubes (even though the age-related impairment in myotube formation is not fully reversed): this evidence further supports adapted physical exercise as a powerful, non-pharmacological approach to counteract sarcopenia and the age-related deterioration of satellite cell capabilities even at very advanced age.
Keywords: immunocytochemistry; sarcopenia; satellite cells; skeletal muscle; treadmill; ultrastructure.
© 2016 Anatomical Society.
Figures

Similar articles
-
Molecular basis of the myogenic profile of aged human skeletal muscle satellite cells during differentiation.Exp Gerontol. 2009 Aug;44(8):523-31. doi: 10.1016/j.exger.2009.05.002. Epub 2009 May 18. Exp Gerontol. 2009. PMID: 19457451
-
Prolonged absence of myostatin reduces sarcopenia.J Cell Physiol. 2006 Dec;209(3):866-73. doi: 10.1002/jcp.20778. J Cell Physiol. 2006. PMID: 16972257
-
Moderate-intensity treadmill running promotes expansion of the satellite cell pool in young and old mice.FEBS J. 2013 Sep;280(17):4063-73. doi: 10.1111/febs.12228. Epub 2013 Apr 12. FEBS J. 2013. PMID: 23464362 Free PMC article.
-
Regulation of microRNAs in Satellite Cell Renewal, Muscle Function, Sarcopenia and the Role of Exercise.Int J Mol Sci. 2020 Sep 14;21(18):6732. doi: 10.3390/ijms21186732. Int J Mol Sci. 2020. PMID: 32937893 Free PMC article. Review.
-
Skeletal muscle degeneration and regeneration in mice and flies.Curr Top Dev Biol. 2014;108:247-81. doi: 10.1016/B978-0-12-391498-9.00007-3. Curr Top Dev Biol. 2014. PMID: 24512712 Review.
Cited by
-
Molecular mechanisms of exercise contributing to tissue regeneration.Signal Transduct _target Ther. 2022 Nov 30;7(1):383. doi: 10.1038/s41392-022-01233-2. Signal Transduct _target Ther. 2022. PMID: 36446784 Free PMC article. Review.
-
Regulation of Satellite Cells Functions during Skeletal Muscle Regeneration: A Critical Step in Physiological and Pathological Conditions.Int J Mol Sci. 2023 Dec 29;25(1):512. doi: 10.3390/ijms25010512. Int J Mol Sci. 2023. PMID: 38203683 Free PMC article. Review.
-
Molecular and Structural Alterations of Skeletal Muscle Tissue Nuclei during Aging.Int J Mol Sci. 2024 Feb 2;25(3):1833. doi: 10.3390/ijms25031833. Int J Mol Sci. 2024. PMID: 38339110 Free PMC article. Review.
-
Satellite Cells in Skeletal Muscle of the Hibernating Dormouse, a Natural Model of Quiescence and Re-Activation: Focus on the Cell Nucleus.Cells. 2020 Apr 23;9(4):1050. doi: 10.3390/cells9041050. Cells. 2020. PMID: 32340154 Free PMC article.
-
Biophysical Stimulation for Engineering Functional Skeletal Muscle.Tissue Eng Part B Rev. 2017 Aug;23(4):362-372. doi: 10.1089/ten.TEB.2016.0444. Tissue Eng Part B Rev. 2017. PMID: 28401807 Free PMC article.
References
-
- Allen RE, Sheehan SM, Taylor RG, et al. (1995) Hepatocyte growth factor activates quiescent skeletal muscle satellite cells in vitro. J Cell Physiol 165, 307–312. - PubMed
-
- Alsharidah M, Lazarus NR, George TE, et al. (2013) Primary human muscle precursor cells obtained from young and old donors produce similar proliferative, differentiation and senescent profiles in culture. Aging Cell 12, 333–344. - PubMed
-
- Anderson JE, Wozniak AC (2004) SC activation on fibers: modeling events in vivo – an invited review. Can J Physiol Pharmacol 82, 300–310. - PubMed
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

