Legionella pneumophila S1P-lyase _targets host sphingolipid metabolism and restrains autophagy
- PMID: 26831115
- PMCID: PMC4763766
- DOI: 10.1073/pnas.1522067113
Legionella pneumophila S1P-lyase _targets host sphingolipid metabolism and restrains autophagy
Abstract
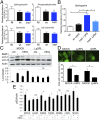
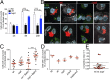
Autophagy is an essential component of innate immunity, enabling the detection and elimination of intracellular pathogens. Legionella pneumophila, an intracellular pathogen that can cause a severe pneumonia in humans, is able to modulate autophagy through the action of effector proteins that are translocated into the host cell by the pathogen's Dot/Icm type IV secretion system. Many of these effectors share structural and sequence similarity with eukaryotic proteins. Indeed, phylogenetic analyses have indicated their acquisition by horizontal gene transfer from a eukaryotic host. Here we report that L. pneumophila translocates the effector protein sphingosine-1 phosphate lyase (LpSpl) to _target the host sphingosine biosynthesis and to curtail autophagy. Our structural characterization of LpSpl and its comparison with human SPL reveals high structural conservation, thus supporting prior phylogenetic analysis. We show that LpSpl possesses S1P lyase activity that was abrogated by mutation of the catalytic site residues. L. pneumophila triggers the reduction of several sphingolipids critical for macrophage function in an LpSpl-dependent and -independent manner. LpSpl activity alone was sufficient to prevent an increase in sphingosine levels in infected host cells and to inhibit autophagy during macrophage infection. LpSpl was required for efficient infection of A/J mice, highlighting an important virulence role for this effector. Thus, we have uncovered a previously unidentified mechanism used by intracellular pathogens to inhibit autophagy, namely the disruption of host sphingolipid biosynthesis.
Keywords: Legionella pneumophila; autophagy; sphingolipids; sphingosine-1-phosphate lyase; virulence.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


Similar articles
-
Legionella pneumophila restrains autophagy by modulating the host's sphingolipid metabolism.Autophagy. 2016 Jun 2;12(6):1053-4. doi: 10.1080/15548627.2016.1166325. Epub 2016 May 18. Autophagy. 2016. PMID: 27191778 Free PMC article.
-
The Sphingosine-1-Phosphate Lyase (LegS2) Contributes to the Restriction of Legionella pneumophila in Murine Macrophages.PLoS One. 2016 Jan 7;11(1):e0146410. doi: 10.1371/journal.pone.0146410. eCollection 2016. PLoS One. 2016. PMID: 26741365 Free PMC article.
-
MAMs are attractive _targets for bacterial repurposing of the host cell: MAM-functions might be key for undermining an infected cell.Bioessays. 2017 Feb;39(2). doi: 10.1002/bies.201600171. Epub 2016 Dec 27. Bioessays. 2017. PMID: 28026026 Review.
-
A Legionella effector acquired from protozoa is involved in sphingolipids metabolism and is _targeted to the host cell mitochondria.Cell Microbiol. 2009 Aug;11(8):1219-35. doi: 10.1111/j.1462-5822.2009.01328.x. Epub 2009 Apr 27. Cell Microbiol. 2009. PMID: 19438520
-
Cell biology and immunology lessons taught by Legionella pneumophila.Sci China Life Sci. 2016 Jan;59(1):3-10. doi: 10.1007/s11427-015-4945-x. Epub 2015 Nov 23. Sci China Life Sci. 2016. PMID: 26596966 Review.
Cited by
-
Mitochondria-Associated Endoplasmic Reticulum Membranes: Inextricably Linked with Autophagy Process.Oxid Med Cell Longev. 2022 Aug 23;2022:7086807. doi: 10.1155/2022/7086807. eCollection 2022. Oxid Med Cell Longev. 2022. PMID: 36052160 Free PMC article. Review.
-
Acanthamoeba and Dictyostelium as Cellular Models for Legionella Infection.Front Cell Infect Microbiol. 2018 Mar 2;8:61. doi: 10.3389/fcimb.2018.00061. eCollection 2018. Front Cell Infect Microbiol. 2018. PMID: 29552544 Free PMC article. Review.
-
Sphingosine kinase 1-associated autophagy differs between neurons and astrocytes.Cell Death Dis. 2018 May 1;9(5):521. doi: 10.1038/s41419-018-0599-5. Cell Death Dis. 2018. PMID: 29743513 Free PMC article.
-
Interaction between autophagic vesicles and the Coxiella-containing vacuole requires CLTC (clathrin heavy chain).Autophagy. 2018;14(10):1710-1725. doi: 10.1080/15548627.2018.1483806. Epub 2018 Jul 29. Autophagy. 2018. PMID: 29973118 Free PMC article.
-
Eating the unknown: Xenophagy and ER-phagy are cytoprotective defenses against pathogens.Exp Cell Res. 2020 Nov 1;396(1):112276. doi: 10.1016/j.yexcr.2020.112276. Epub 2020 Sep 9. Exp Cell Res. 2020. PMID: 32918896 Free PMC article. Review.
References
-
- Escoll P, Rolando M, Gomez-Valero L, Buchrieser C. From amoeba to macrophages: Exploring the molecular mechanisms of Legionella pneumophila infection in both hosts. Curr Top Microbiol Immunol. 2013;376:1–34. - PubMed
-
- Cazalet C, et al. Evidence in the Legionella pneumophila genome for exploitation of host cell functions and high genome plasticity. Nat Genet. 2004;36(11):1165–1173. - PubMed
-
- Degtyar E, Zusman T, Ehrlich M, Segal G. A Legionella effector acquired from protozoa is involved in sphingolipids metabolism and is _targeted to the host cell mitochondria. Cell Microbiol. 2009;11(8):1219–1235. - PubMed
Publication types
MeSH terms
Substances
Associated data
- Actions
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

