Molecular optimization of rabies virus glycoprotein expression in Pichia pastoris
- PMID: 26880068
- PMCID: PMC4835572
- DOI: 10.1111/1751-7915.12350
Molecular optimization of rabies virus glycoprotein expression in Pichia pastoris
Abstract
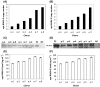
In this work, different approaches were investigated to enhance the expression rabies virus glycoprotein (RABV-G) in the yeast Pichia pastoris; this membrane protein is responsible for the synthesis of rabies neutralizing antibodies. First, the impact of synonymous codon usage bias was examined and an optimized RABV-G gene was synthesized. Nevertheless, data showed that the secretion of the optimized RABV-G gene was not tremendously increased as compared with the non-optimized one. In addition, similar levels of RABV-G were obtained when α-factor mating factor from Saccharomyces cerevisiae or the acid phosphatase PHO1 was used as a secretion signal. Therefore, sequence optimization and secretion signal were not the major bottlenecks for high-level expression of RABV-G in P. pastoris. Unfolded protein response (UPR) was induced in clones containing high copy number of RABV-G expression cassette indicating that folding was the limiting step for RABV-G secretion. To circumvent this limitation, co-overexpression of five factors involved in oxidative protein folding was investigated. Among these factors only PDI1, ERO1 and GPX1 proved their benefit to enhance the expression. The highest expression level of RABV-G reached 1230 ng ml(-1). Competitive neutralizing assay confirmed that the recombinant protein was produced in the correct conformational form in this host.
© 2016 The Authors. Microbial Biotechnology published by John Wiley & Sons Ltd and Society for Applied Microbiology.
Figures
Similar articles
-
Investigating the effect of carbon source on rabies virus glycoprotein production in Pichia pastoris by a transcriptomic approach.Microbiologyopen. 2017 Aug;6(4):e00489. doi: 10.1002/mbo3.489. Epub 2017 May 18. Microbiologyopen. 2017. PMID: 28523730 Free PMC article.
-
Engineering of the unfolded protein response pathway in Pichia pastoris: enhancing production of secreted recombinant proteins.Appl Microbiol Biotechnol. 2021 Jun;105(11):4397-4414. doi: 10.1007/s00253-021-11336-5. Epub 2021 May 26. Appl Microbiol Biotechnol. 2021. PMID: 34037840 Free PMC article. Review.
-
Expression of rabies virus glycoprotein in the methylotrophic yeast Pichia pastoris.Biotechnol Appl Biochem. 2017 Jan;64(1):50-61. doi: 10.1002/bab.1471. Epub 2016 Apr 14. Biotechnol Appl Biochem. 2017. PMID: 28218973
-
Controlled viral glycoprotein expression as a safety feature in a bivalent rabies-ebola vaccine.Virus Res. 2015 Feb 2;197:54-8. doi: 10.1016/j.virusres.2014.11.028. Epub 2014 Dec 4. Virus Res. 2015. PMID: 25481284 Free PMC article.
-
Recent advances in the production of recombinant subunit vaccines in Pichia pastoris.Bioengineered. 2016 Apr;7(3):155-65. doi: 10.1080/21655979.2016.1191707. Bioengineered. 2016. PMID: 27246656 Free PMC article. Review.
Cited by
-
Expanding the promoter toolbox for metabolic engineering of methylotrophic yeasts.Appl Microbiol Biotechnol. 2022 May;106(9-10):3449-3464. doi: 10.1007/s00253-022-11948-5. Epub 2022 May 11. Appl Microbiol Biotechnol. 2022. PMID: 35538374 Review.
-
NS1 Recombinant Proteins Are Efficiently Produced in Pichia pastoris and Have Great Potential for Use in Diagnostic Kits for Dengue Virus Infections.Diagnostics (Basel). 2020 Jun 6;10(6):379. doi: 10.3390/diagnostics10060379. Diagnostics (Basel). 2020. PMID: 32517281 Free PMC article.
-
Investigating the effect of carbon source on rabies virus glycoprotein production in Pichia pastoris by a transcriptomic approach.Microbiologyopen. 2017 Aug;6(4):e00489. doi: 10.1002/mbo3.489. Epub 2017 May 18. Microbiologyopen. 2017. PMID: 28523730 Free PMC article.
-
Alternative PCR-Based Approaches for Generation of Komagataella phaffii Strains.Microorganisms. 2023 Sep 12;11(9):2297. doi: 10.3390/microorganisms11092297. Microorganisms. 2023. PMID: 37764140 Free PMC article.
-
Engineering of the unfolded protein response pathway in Pichia pastoris: enhancing production of secreted recombinant proteins.Appl Microbiol Biotechnol. 2021 Jun;105(11):4397-4414. doi: 10.1007/s00253-021-11336-5. Epub 2021 May 26. Appl Microbiol Biotechnol. 2021. PMID: 34037840 Free PMC article. Review.
References
-
- Anilionis, A. , Wunner, W.H. , and Curtis, P.J. (1981) Structure of the glycoprotein gene in rabies virus. Nature, 294: 275–278. - PubMed
-
- Ashe, M.P. , and Bill, R.M. (2011) Mapping the yeast host cell response to recombinant membrane protein production relieving the biological bottlenecks. Biotechnol J, 6: 707–714. - PubMed
Publication types
MeSH terms
Substances
Associated data
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

