AMPK antagonizes hepatic glucagon-stimulated cyclic AMP signalling via phosphorylation-induced activation of cyclic nucleotide phosphodiesterase 4B
- PMID: 26952277
- PMCID: PMC4786776
- DOI: 10.1038/ncomms10856
AMPK antagonizes hepatic glucagon-stimulated cyclic AMP signalling via phosphorylation-induced activation of cyclic nucleotide phosphodiesterase 4B
Abstract
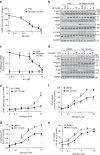
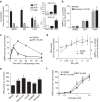
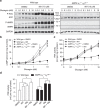
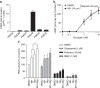
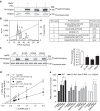
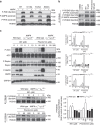
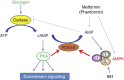
Biguanides such as metformin have previously been shown to antagonize hepatic glucagon-stimulated cyclic AMP (cAMP) signalling independently of AMP-activated protein kinase (AMPK) via direct inhibition of adenylate cyclase by AMP. Here we show that incubation of hepatocytes with the small-molecule AMPK activator 991 decreases glucagon-stimulated cAMP accumulation, cAMP-dependent protein kinase (PKA) activity and downstream PKA _target phosphorylation. Moreover, incubation of hepatocytes with 991 increases the Vmax of cyclic nucleotide phosphodiesterase 4B (PDE4B) without affecting intracellular adenine nucleotide concentrations. The effects of 991 to decrease glucagon-stimulated cAMP concentrations and activate PDE4B are lost in hepatocytes deleted for both catalytic subunits of AMPK. PDE4B is phosphorylated by AMPK at three sites, and by site-directed mutagenesis, Ser304 phosphorylation is important for activation. In conclusion, we provide a new mechanism by which AMPK antagonizes hepatic glucagon signalling via phosphorylation-induced PDE4B activation.
Figures
Similar articles
-
Biguanides suppress hepatic glucagon signalling by decreasing production of cyclic AMP.Nature. 2013 Feb 14;494(7436):256-60. doi: 10.1038/nature11808. Epub 2013 Jan 6. Nature. 2013. PMID: 23292513 Free PMC article.
-
Differential AMPK phosphorylation by glucagon and metformin regulates insulin signaling in human hepatic cells.Biochem Biophys Res Commun. 2014 May 16;447(4):569-73. doi: 10.1016/j.bbrc.2014.04.031. Epub 2014 Apr 13. Biochem Biophys Res Commun. 2014. PMID: 24735537
-
Regulation of AMP-activated protein kinase by cAMP in adipocytes: roles for phosphodiesterases, protein kinase B, protein kinase A, Epac and lipolysis.Cell Signal. 2009 May;21(5):760-6. doi: 10.1016/j.cellsig.2009.01.015. Epub 2009 Jan 8. Cell Signal. 2009. PMID: 19167487 Free PMC article.
-
Disrupted in schizophrenia 1 and phosphodiesterase 4B: towards an understanding of psychiatric illness.J Physiol. 2007 Oct 15;584(Pt 2):401-5. doi: 10.1113/jphysiol.2007.140210. Epub 2007 Sep 6. J Physiol. 2007. PMID: 17823207 Free PMC article. Review.
-
Emerging Role of cAMP/AMPK Signaling.Cells. 2022 Jan 17;11(2):308. doi: 10.3390/cells11020308. Cells. 2022. PMID: 35053423 Free PMC article. Review.
Cited by
-
Cellular and Molecular Mechanisms of Metformin Action.Endocr Rev. 2021 Jan 28;42(1):77-96. doi: 10.1210/endrev/bnaa023. Endocr Rev. 2021. PMID: 32897388 Free PMC article. Review.
-
Modulation of cardiac cAMP signaling by AMPK and its adjustments in pressure overload-induced myocardial dysfunction in rat and mouse.PLoS One. 2023 Sep 21;18(9):e0292015. doi: 10.1371/journal.pone.0292015. eCollection 2023. PLoS One. 2023. PMID: 37733758 Free PMC article.
-
Spinal AMP kinase activity differentially regulates phrenic motor plasticity.J Appl Physiol (1985). 2020 Mar 1;128(3):523-533. doi: 10.1152/japplphysiol.00546.2019. Epub 2020 Jan 23. J Appl Physiol (1985). 2020. PMID: 31971473 Free PMC article.
-
Roles of protein post-translational modifications in glucose and lipid metabolism: mechanisms and perspectives.Mol Med. 2023 Jul 6;29(1):93. doi: 10.1186/s10020-023-00684-9. Mol Med. 2023. PMID: 37415097 Free PMC article. Review.
-
Nicotinamide Treatment Facilitates Mitochondrial Fission through Drp1 Activation Mediated by SIRT1-Induced Changes in Cellular Levels of cAMP and Ca2.Cells. 2021 Mar 10;10(3):612. doi: 10.3390/cells10030612. Cells. 2021. PMID: 33802063 Free PMC article.
References
-
- Exton J. H., Robison G. A., Sutherland E. W. & Park C. R. Studies on the role of adenosine 3′,5′-monophosphate in the hepatic actions of glucagon and catecholamines. J. Biol. Chem. 246, 6166–6177 (1971). - PubMed
-
- Pilkis S., Schlumpf J., Pilkis J. & Claus T. H. Regulation of phosphofructokinase activity by glucagon in isolated rat hepatocytes. Biochem. Biophys. Res. Commun. 88, 960–967 (1979). - PubMed
-
- Hanson R. W. & Mehlman M. A. Gluconeogenesis: Its Regulation in Mammalian Species 515–532Wiley (1976).
-
- Sutherland E. W. Studies on the mechanism of hormone action. Science 177, 401–408 (1972). - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

