Lipopolysaccharide and Tumor Necrosis Factor Alpha Inhibit Interferon Signaling in Hepatocytes by Increasing Ubiquitin-Like Protease 18 (USP18) Expression
- PMID: 27009955
- PMCID: PMC4886784
- DOI: 10.1128/JVI.02557-15
Lipopolysaccharide and Tumor Necrosis Factor Alpha Inhibit Interferon Signaling in Hepatocytes by Increasing Ubiquitin-Like Protease 18 (USP18) Expression
Abstract
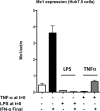
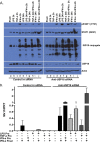
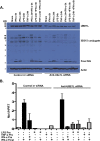
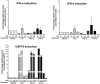
Inflammation may be maladaptive to the control of viral infection when it impairs interferon (IFN) responses, enhancing viral replication and spread. Dysregulated immunity as a result of inappropriate innate inflammatory responses is a hallmark of chronic viral infections such as, hepatitis B virus and hepatitis C virus (HCV). Previous studies from our laboratory have shown that expression of an IFN-stimulated gene (ISG), ubiquitin-like protease (USP)18 is upregulated in chronic HCV infection, leading to impaired hepatocyte responses to IFN-α. We examined the ability of inflammatory stimuli, including tumor necrosis factor alpha (TNF-α), lipopolysaccharide (LPS), interleukin-6 (IL-6) and IL-10 to upregulate hepatocyte USP18 expression and blunt the IFN-α response. Human hepatoma cells and primary murine hepatocytes were treated with TNF-α/LPS/IL-6/IL-10 and USP18, phosphorylated (p)-STAT1 and myxovirus (influenza virus) resistance 1 (Mx1) expression was determined. Treatment of Huh7.5 cells and primary murine hepatocytes with LPS and TNF-α, but not IL-6 or IL-10, led to upregulated USP18 expression and induced an IFN-α refractory state, which was reversed by USP18 knockdown. Liver inflammation was induced in vivo using a murine model of hepatic ischemia/reperfusion injury. Hepatic ischemia/reperfusion injury led to an induction of USP18 expression in liver tissue and promotion of lymphocytic choriomeningitis replication. These data demonstrate that certain inflammatory stimuli (TNF-α and LPS) but not others (IL-6 and IL-10) _target USP18 expression and thus inhibit IFN signaling. These findings represent a new paradigm for how inflammation alters hepatic innate immune responses, with USP18 representing a potential _target for intervention in various inflammatory states.
Importance: Inflammation may prevent the control of viral infection when it impairs the innate immune response, enhancing viral replication and spread. Blunted immunity as a result of inappropriate innate inflammatory responses is a common characteristic of chronic viral infections. Previous studies have shown that expression of certain interferon-stimulated genes is upregulated in chronic HCV infection, leading to impaired hepatocyte responses. In this study, we show that multiple inflammatory stimuli can modulate interferon stimulated gene expression and thus inhibit hepatocyte interferon signaling via USP18 induction. These findings represent a new paradigm for how inflammation alters hepatic innate immune responses, with the induction of USP18 representing a potential _target for intervention in various inflammatory states.
Copyright © 2016 MacParland et al.
Figures

Similar articles
-
Acetaldehyde Disrupts Interferon Alpha Signaling in Hepatitis C Virus-Infected Liver Cells by Up-Regulating USP18.Alcohol Clin Exp Res. 2016 Nov;40(11):2329-2338. doi: 10.1111/acer.13226. Epub 2016 Sep 26. Alcohol Clin Exp Res. 2016. PMID: 27716962 Free PMC article.
-
Silencing of USP18 potentiates the antiviral activity of interferon against hepatitis C virus infection.Gastroenterology. 2006 Nov;131(5):1584-91. doi: 10.1053/j.gastro.2006.08.043. Epub 2006 Aug 22. Gastroenterology. 2006. PMID: 17101330
-
Alcohol impairs interferon signaling and enhances full cycle hepatitis C virus JFH-1 infection of human hepatocytes.Drug Alcohol Depend. 2010 Nov 1;112(1-2):107-16. doi: 10.1016/j.drugalcdep.2010.05.008. Epub 2010 Jun 20. Drug Alcohol Depend. 2010. PMID: 20646875 Free PMC article.
-
The ISG15/USP18 ubiquitin-like pathway (ISGylation system) in hepatitis C virus infection and resistance to interferon therapy.Int J Biochem Cell Biol. 2011 Oct;43(10):1427-31. doi: 10.1016/j.biocel.2011.06.006. Epub 2011 Jun 16. Int J Biochem Cell Biol. 2011. PMID: 21704181 Review.
-
Decoding cell death signals in liver inflammation.J Hepatol. 2013 Sep;59(3):583-94. doi: 10.1016/j.jhep.2013.03.033. Epub 2013 Apr 6. J Hepatol. 2013. PMID: 23567086 Review.
Cited by
-
Multiple functions of USP18.Cell Death Dis. 2016 Nov 3;7(11):e2444. doi: 10.1038/cddis.2016.326. Cell Death Dis. 2016. PMID: 27809302 Free PMC article. Review.
-
The diverse repertoire of ISG15: more intricate than initially thought.Exp Mol Med. 2022 Nov;54(11):1779-1792. doi: 10.1038/s12276-022-00872-3. Epub 2022 Nov 1. Exp Mol Med. 2022. PMID: 36319753 Free PMC article. Review.
-
Relationship Between Ubiquitin-Specific Peptidase 18 and Hypertension in Polish Adult Male Subjects: A Cross-Sectional Pilot Study.Med Sci Monit. 2020 Jun 12;26:e921919. doi: 10.12659/MSM.921919. Med Sci Monit. 2020. PMID: 32527992 Free PMC article.
-
Suppression of USP18 Potentiates the Anti-HBV Activity of Interferon Alpha in HepG2.2.15 Cells via JAK/STAT Signaling.PLoS One. 2016 May 26;11(5):e0156496. doi: 10.1371/journal.pone.0156496. eCollection 2016. PLoS One. 2016. PMID: 27227879 Free PMC article.
-
MicroRNA-146a regulates the transformation from liver fibrosis to cirrhosis in patients with hepatitis B via interleukin-6.Exp Ther Med. 2019 Jun;17(6):4670-4676. doi: 10.3892/etm.2019.7490. Epub 2019 Apr 16. Exp Ther Med. 2019. PMID: 31086599 Free PMC article.
References
-
- Nishitsuji H, Funami K, Shimizu Y, Ujino S, Sugiyama K, Seya T, Takaku H, Shimotohno K. 2013. Hepatitis C virus infection induces inflammatory cytokines and chemokines mediated by the cross talk between hepatocytes and stellate cells. J Virol 87:8169–8178. doi:10.1128/JVI.00974-13. - DOI - PMC - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

