Wnt/β-catenin signaling plays an ever-expanding role in stem cell self-renewal, tumorigenesis and cancer chemoresistance
- PMID: 27077077
- PMCID: PMC4827448
- DOI: 10.1016/j.gendis.2015.12.004
Wnt/β-catenin signaling plays an ever-expanding role in stem cell self-renewal, tumorigenesis and cancer chemoresistance
Abstract
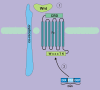
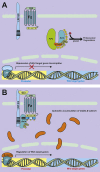
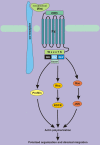
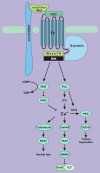
Wnt signaling transduces evolutionarily conserved pathways which play important roles in initiating and regulating a diverse range of cellular activities, including cell proliferation, calcium homeostasis, and cell polarity. The role of Wnt signaling in control of cell proliferation and stem cell self-renewal is primarily carried out through the canonical pathway, which is the best characterized among the multiple Wnt signaling branches. The past 10 years has seen a rapid expansion in our understanding of the complexity of this pathway, as many new components of Wnt signaling have been identified and linked to signaling regulation, stem cell functions, and adult tissue homeostasis. Additionally, a substantial body of evidence links Wnt signaling to tumorigenesis of many cancer types and implicates it in the development of cancer drug resistance. Thus, a better understanding of the mechanisms by which dysregulation of Wnt signaling precedes the development and progression of human cancer may hasten the development of pathway inhibitors to augment current therapy. This review summarizes and synthesizes our current knowledge of the canonical Wnt pathway in development and disease. We begin with an overview of the components of the canonical Wnt signaling pathway and delve into the role this pathway has been shown to play in stemness, tumorigenesis, and cancer drug resistance. Ultimately, we hope to present an organized collection of evidence implicating Wnt signaling in tumorigenesis and chemoresistance to facilitate the pursuit of Wnt pathway modulators that may improve outcomes of cancers in which Wnt signaling contributes to aggressive disease and/or treatment resistance.
Keywords: Wnt; cancer; cancer drug resistance; cancer stem cells; canonical Wnt; cell differentiation; cell proliferation; fate determination; self-renewal; signal transduction; stem cells; tumorigenesis; β-catenin.
Figures
Similar articles
-
The evolving roles of Wnt signaling in stem cell proliferation and differentiation, the development of human diseases, and therapeutic opportunities.Genes Dis. 2023 Jul 22;11(3):101026. doi: 10.1016/j.gendis.2023.04.042. eCollection 2024 May. Genes Dis. 2023. PMID: 38292186 Free PMC article. Review.
-
Noncanonical Wnt signaling plays an important role in modulating canonical Wnt-regulated stemness, proliferation and terminal differentiation of hepatic progenitors.Onco_target. 2017 Apr 18;8(16):27105-27119. doi: 10.18632/onco_target.15637. Onco_target. 2017. PMID: 28404920 Free PMC article.
-
Frizzled7 Promotes Epithelial-to-mesenchymal Transition and Stemness Via Activating Canonical Wnt/β-catenin Pathway in Gastric Cancer.Int J Biol Sci. 2018 Feb 12;14(3):280-293. doi: 10.7150/ijbs.23756. eCollection 2018. Int J Biol Sci. 2018. PMID: 29559846 Free PMC article.
-
New Advances in Canonical Wnt/β-Catenin Signaling in Cancer.Cancer Manag Res. 2020 Aug 6;12:6987-6998. doi: 10.2147/CMAR.S258645. eCollection 2020. Cancer Manag Res. 2020. PMID: 32821165 Free PMC article. Review.
-
Promising Druggable _target in Head and Neck Squamous Cell Carcinoma: Wnt Signaling.Front Pharmacol. 2016 Aug 12;7:244. doi: 10.3389/fphar.2016.00244. eCollection 2016. Front Pharmacol. 2016. PMID: 27570510 Free PMC article. Review.
Cited by
-
The Role of Tumor Stem Cells in Colorectal Cancer Drug Resistance.Cancer Control. 2024 Jan-Dec;31:10732748241274196. doi: 10.1177/10732748241274196. Cancer Control. 2024. PMID: 39215442 Free PMC article. Review.
-
m6A modification of CDC5L promotes lung adenocarcinoma progression through transcriptionally regulating WNT7B expression.Am J Cancer Res. 2024 Jul 15;14(7):3565-3583. doi: 10.62347/QHFA9669. eCollection 2024. Am J Cancer Res. 2024. PMID: 39113868 Free PMC article.
-
Solid pseudopapillary neoplasm (SPN) of the pancreas: current understanding on its malignant potential and management.Discov Oncol. 2024 Mar 18;15(1):77. doi: 10.1007/s12672-024-00905-5. Discov Oncol. 2024. PMID: 38498246 Free PMC article. Review.
-
Endothelial β-catenin upregulation and Y142 phosphorylation drive diabetic angiogenesis via upregulating KDR/HDAC9.Cell Commun Signal. 2024 Mar 15;22(1):182. doi: 10.1186/s12964-024-01566-1. Cell Commun Signal. 2024. PMID: 38491522 Free PMC article.
-
MicroRNA-204 Regulates Angiogenesis and Vasculogenic Mimicry in CD44+/CD24- Breast Cancer Stem-like Cells.Noncoding RNA. 2024 Feb 9;10(1):14. doi: 10.3390/ncrna10010014. Noncoding RNA. 2024. PMID: 38392969 Free PMC article.
References
-
- Clevers H., Loh K.M., Nusse R. Stem cell signaling. An integral program for tissue renewal and regeneration: Wnt signaling and stem cell control. Science. 2014;346:1248012. - PubMed
-
- Niehrs C. The complex world of WNT receptor signalling. Nat Rev Mol Cell Biol. 2012;13:767–779. - PubMed
-
- Anastas J.N., Moon R.T. WNT signalling pathways as therapeutic _targets in cancer. Nat Rev Cancer. 2013;13:11–26. - PubMed
-
- Luo J., Chen J., Deng Z.-L. Wnt signaling and human diseases: what are the therapeutic implications? Lab Investig J Tech Methods Pathol. 2007;87:97–103. - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous
