Ventromedial hypothalamic melanocortin receptor activation: regulation of activity energy expenditure and skeletal muscle thermogenesis
- PMID: 27126579
- PMCID: PMC5023712
- DOI: 10.1113/JP272352
Ventromedial hypothalamic melanocortin receptor activation: regulation of activity energy expenditure and skeletal muscle thermogenesis
Abstract
Key points: The ventromedial hypothalamus (VMH) and the central melanocortin system both play vital roles in regulating energy balance by modulating energy intake and utilization. Recent evidence suggests that activation of the VMH alters skeletal muscle metabolism. We show that intra-VMH melanocortin receptor activation increases energy expenditure and physical activity, switches fuel utilization to fats, and lowers work efficiency such that excess calories are dissipated by skeletal muscle as heat. We also show that intra-VMH melanocortin receptor activation increases sympathetic nervous system outflow to skeletal muscle. Intra-VMH melanocortin receptor activation also induced significant changes in the expression of mediators of energy expenditure in muscle. These results support the role of melanocortin receptors in the VMH in the modulation of skeletal muscle metabolism.
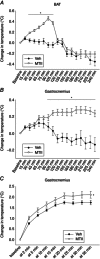
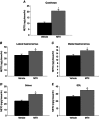
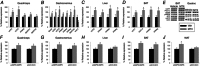
Abstract: The ventromedial hypothalamus (VMH) and the brain melanocortin system both play vital roles in increasing energy expenditure (EE) and physical activity, decreasing appetite and modulating sympathetic nervous system (SNS) outflow. Because of recent evidence showing that VMH activation modulates skeletal muscle metabolism, we propose the existence of an axis between the VMH and skeletal muscle, modulated by brain melanocortins, modelled on the brain control of brown adipose tissue. Activation of melanocortin receptors in the VMH of rats using a non-specific agonist melanotan II (MTII), compared to vehicle, increased oxygen consumption and EE and decreased the respiratory exchange ratio. Intra-VMH MTII enhanced activity-related EE even when activity levels were held constant. MTII treatment increased gastrocnemius muscle heat dissipation during controlled activity, as well as in the home cage. Compared to vehicle-treated rats, rats with intra-VMH melanocortin receptor activation had higher skeletal muscle norepinephrine turnover, indicating an increased SNS drive to muscle. Lastly, intra-VMH MTII induced mRNA expression of muscle energetic mediators, whereas short-term changes at the protein level were primarily limited to phosphorylation events. These results support the hypothesis that melanocortin peptides act in the VMH to increase EE by lowering the economy of activity via the enhanced expression of mediators of EE in the periphery including skeletal muscle. The data are consistent with the role of melanocortins in the VMH in the modulation of skeletal muscle metabolism.
© 2016 The Authors. The Journal of Physiology © 2016 The Physiological Society.
Figures


Comment in
-
How the brain tips the scale.J Physiol. 2016 Sep 15;594(18):5041-2. doi: 10.1113/JP272701. J Physiol. 2016. PMID: 27629075 Free PMC article. No abstract available.
Similar articles
-
Differential activation of the sympathetic innervation of adipose tissues by melanocortin receptor stimulation.Endocrinology. 2007 Nov;148(11):5339-47. doi: 10.1210/en.2007-0621. Epub 2007 Aug 16. Endocrinology. 2007. PMID: 17702843
-
Inherently Lean Rats Have Enhanced Activity and Skeletal Muscle Response to Central Melanocortin Receptors.Obesity (Silver Spring). 2018 May;26(5):885-894. doi: 10.1002/oby.22166. Epub 2018 Mar 22. Obesity (Silver Spring). 2018. PMID: 29566460 Free PMC article.
-
Suppressed sympathetic outflow to skeletal muscle, muscle thermogenesis, and activity energy expenditure with calorie restriction.Physiol Rep. 2017 Feb;5(4):e13171. doi: 10.14814/phy2.13171. Epub 2017 Feb 27. Physiol Rep. 2017. PMID: 28242830 Free PMC article.
-
Hypothalamic-autonomic control of energy homeostasis.Endocrine. 2015 Nov;50(2):276-91. doi: 10.1007/s12020-015-0658-y. Epub 2015 Jun 19. Endocrine. 2015. PMID: 26089260 Review.
-
Energy expenditure: a critical determinant of energy balance with key hypothalamic controls.Minerva Endocrinol. 2007 Sep;32(3):173-83. Minerva Endocrinol. 2007. PMID: 17912156 Review.
Cited by
-
Aerobic capacity modulates adaptive thermogenesis: Contribution of non-resting energy expenditure.Physiol Behav. 2020 Oct 15;225:113048. doi: 10.1016/j.physbeh.2020.113048. Epub 2020 Jul 3. Physiol Behav. 2020. PMID: 32628949 Free PMC article.
-
Control of Energy Expenditure by AgRP Neurons of the Arcuate Nucleus: Neurocircuitry, Signaling Pathways, and Angiotensin.Curr Hypertens Rep. 2018 Mar 19;20(3):25. doi: 10.1007/s11906-018-0824-8. Curr Hypertens Rep. 2018. PMID: 29556733 Free PMC article. Review.
-
Brown adipocyte and browning thermogenesis: metabolic crosstalk beyond mitochondrial limits and physiological impacts.Adipocyte. 2023 Dec;12(1):2237164. doi: 10.1080/21623945.2023.2237164. Adipocyte. 2023. PMID: 37488770 Free PMC article. Review.
-
Exercise, Obesity and CNS Control of Metabolic Homeostasis: A Review.Front Physiol. 2018 May 17;9:574. doi: 10.3389/fphys.2018.00574. eCollection 2018. Front Physiol. 2018. PMID: 29867590 Free PMC article. Review.
-
Ventromedial hypothalamic nucleus neuronal subset regulates blood glucose independently of insulin.J Clin Invest. 2020 Jun 1;130(6):2943-2952. doi: 10.1172/JCI134135. J Clin Invest. 2020. PMID: 32134398 Free PMC article.
References
-
- Takahashi Akira & Shimazu Takashi (1981). Hypothalamic regulation of lipid metabolism in the rat: effect of hypothalamic stimulation on lipolysis. J Auton Nerv Syst 4, 195–205. - PubMed
-
- Anand BK & Brobeck JR (1951). Localization of a “feeding center” in the hypothalamus of the rat. Proc Soc Exp Biol Med 77, 323–324. - PubMed
-
- Belgardt BF & Bruning JC (2010). CNS leptin and insulin action in the control of energy homeostasis. Ann NY Acad Sci 1212, 97–113. - PubMed
-
- Bonen A, Tandon NN, Glatz JF, Luiken JJ & Heigenhauser GJ (2006). The fatty acid transporter FAT/CD36 is upregulated in subcutaneous and visceral adipose tissues in human obesity and type 2 diabetes. Int J Obes (Lond) 30, 877–883. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

