Penfluridol suppresses pancreatic tumor growth by autophagy-mediated apoptosis
- PMID: 27189859
- PMCID: PMC4870635
- DOI: 10.1038/srep26165
Penfluridol suppresses pancreatic tumor growth by autophagy-mediated apoptosis
Abstract
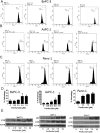
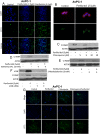
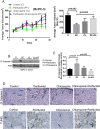
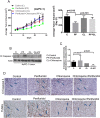
Pancreatic tumors exhibit enhanced autophagy as compared to any other cancer, making it resistant to chemotherapy. We evaluated the effect of penfluridol against pancreatic cancer. Penfluridol treatment induced apoptosis and inhibited the growth of Panc-1, BxPC-3 and AsPC-1, pancreatic cancer cells with IC50 ranging between 6-7 μM after 24 h of treatment. Significant autophagy was induced by penfluridol treatment in pancreatic cancer cells. Punctate LC3B and autophagosomes staining confirmed autophagy. Inhibiting autophagy by chloroquine, bafilomycin, 3-methyladenine or LC3BsiRNA, significantly blocked penfluridol-induced apoptosis, suggesting that autophagy lead to apoptosis in our model. Penfluridol treatment suppressed the growth of BxPC-3 tumor xenografts by 48% as compared to 17% when treated in combination with chloroquine. Similarly, penfluridol suppressed the growth of AsPC-1 tumors by 40% versus 16% when given in combination with chloroquine. TUNEL staining and caspase-3 cleavage revealed less apoptosis in the tumors from mice treated with penfluridol and chloroquine as compared to penfluridol alone. Penfluridol treatment also suppressed the growth of orthotopically implanted Panc-1 tumors by 80% by inducing autophagy-mediated apoptosis in the tumors. These studies established that penfluridol inhibits pancreatic tumor growth by autophagy-mediated apoptosis. Since penfluridol is already in clinic, positive findings from our study will accelerate its clinical development.
Figures


Similar articles
-
Penfluridol induces endoplasmic reticulum stress leading to autophagy in pancreatic cancer.Tumour Biol. 2017 Jun;39(6):1010428317705517. doi: 10.1177/1010428317705517. Tumour Biol. 2017. PMID: 28618969
-
Diphenylbutylpiperidine Antipsychotic Drugs Inhibit Prolactin Receptor Signaling to Reduce Growth of Pancreatic Ductal Adenocarcinoma in Mice.Gastroenterology. 2020 Apr;158(5):1433-1449.e27. doi: 10.1053/j.gastro.2019.11.279. Epub 2019 Nov 29. Gastroenterology. 2020. PMID: 31786131 Free PMC article.
-
Penfluridol suppresses glioblastoma tumor growth by Akt-mediated inhibition of GLI1.Onco_target. 2017 May 16;8(20):32960-32976. doi: 10.18632/onco_target.16515. Onco_target. 2017. PMID: 28380428 Free PMC article.
-
Autophagy Inhibition in Pancreatic Adenocarcinoma.Clin Colorectal Cancer. 2018 Mar;17(1):25-31. doi: 10.1016/j.clcc.2017.10.013. Epub 2017 Oct 28. Clin Colorectal Cancer. 2018. PMID: 29223362 Review.
-
Penfluridol as a Candidate of Drug Repurposing for Anticancer Agent.Molecules. 2019 Oct 11;24(20):3659. doi: 10.3390/molecules24203659. Molecules. 2019. PMID: 31614431 Free PMC article. Review.
Cited by
-
miR-138-5p suppresses autophagy in pancreatic cancer by _targeting SIRT1.Onco_target. 2017 Feb 14;8(7):11071-11082. doi: 10.18632/onco_target.14360. Onco_target. 2017. PMID: 28052003 Free PMC article.
-
Penfluridol inhibits melanoma growth and metastasis through enhancing von Hippel‒Lindau tumor suppressor-mediated cancerous inhibitor of protein phosphatase 2A (CIP2A) degradation.MedComm (2020). 2024 Oct 13;5(10):e758. doi: 10.1002/mco2.758. eCollection 2024 Oct. MedComm (2020). 2024. PMID: 39399646 Free PMC article.
-
Penfluridol overcomes paclitaxel resistance in metastatic breast cancer.Sci Rep. 2019 Mar 25;9(1):5066. doi: 10.1038/s41598-019-41632-0. Sci Rep. 2019. PMID: 30911062 Free PMC article.
-
Exosomes as a Surrogate Marker for Autophagy in Peripheral Blood, Correlative Data from Phase I Study of Chloroquine in Combination with Carboplatin/Gemcitabine in Advanced Solid Tumors.Asian Pac J Cancer Prev. 2019 Dec 1;20(12):3789-3796. doi: 10.31557/APJCP.2019.20.12.3789. Asian Pac J Cancer Prev. 2019. PMID: 31870123 Free PMC article.
-
Low Dose of Penfluridol Inhibits VEGF-Induced Angiogenesis.Int J Mol Sci. 2020 Jan 23;21(3):755. doi: 10.3390/ijms21030755. Int J Mol Sci. 2020. PMID: 31979394 Free PMC article.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

