Cyclic Nucleotide Control of Microtubule Dynamics for Axon Guidance
- PMID: 27194341
- PMCID: PMC6601770
- DOI: 10.1523/JNEUROSCI.3596-15.2016
Cyclic Nucleotide Control of Microtubule Dynamics for Axon Guidance
Abstract
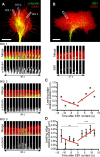
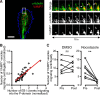
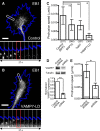
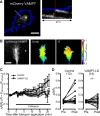
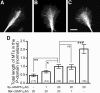
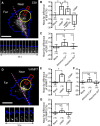
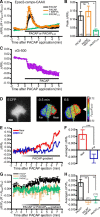
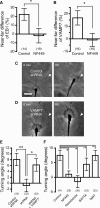
Graded distribution of intracellular second messengers, such as Ca(2+) and cyclic nucleotides, mediates directional cell migration, including axon navigational responses to extracellular guidance cues, in the developing nervous system. Elevated concentrations of cAMP or cGMP on one side of the neuronal growth cone induce its attractive or repulsive turning, respectively. Although effector processes downstream of Ca(2+) have been extensively studied, very little is known about the mechanisms that enable cyclic nucleotides to steer migrating cells. Here, we show that asymmetric cyclic nucleotide signaling across the growth cone mediates axon guidance via modulating microtubule dynamics and membrane organelle transport. In embryonic chick dorsal root ganglion neurons in culture, contact of an extending microtubule with the growth cone leading edge induces localized membrane protrusion at the site of microtubule contact. Such a contact-induced protrusion requires exocytosis of vesicle-associated membrane protein 7 (VAMP7)-positive vesicles that have been transported centrifugally along the microtubule. We found that the two cyclic nucleotides counteractively regulate the frequency of microtubule contacts and _targeted delivery of VAMP7 vesicles: cAMP stimulates and cGMP inhibits these events, thereby steering the growth cone in the opposite directions. By contrast, Ca(2+) signals elicit no detectable change in either microtubule contacts or VAMP7 vesicle delivery during Ca(2+)-induced growth cone turning. Our findings clearly demonstrate growth cone steering machinery downstream of cyclic nucleotide signaling and highlight a crucial role of dynamic microtubules in leading-edge protrusion for cell chemotaxis.
Significance statement: Developing neurons can extend long axons toward their postsynaptic _targets. The tip of each axon, called the growth cone, recognizes extracellular guidance cues and navigates the axon along the correct path. Here we show that asymmetric cyclic nucleotide signaling across the growth cone mediates axon guidance through localized regulation of microtubule dynamics and resulting recruitment of specific populations of membrane vesicles to the growth cone's leading edge. Remarkably, cAMP stimulates microtubule growth and membrane protrusion, whereas cGMP promotes microtubule retraction and membrane senescence, explaining the opposite directional polarities of growth cone turning induced by these cyclic nucleotides. This study reveals a novel microtubule-based mechanism through which cyclic nucleotides polarize the growth cone steering machinery for bidirectional axon guidance.
Keywords: VAMP7; axon guidance; cyclic nucleotide; growth cone; microtubule.
Copyright © 2016 the authors 0270-6474/16/365636-14$15.00/0.
Figures

Similar articles
-
The nitric oxide-cGMP pathway controls the directional polarity of growth cone guidance via modulating cytosolic Ca2+ signals.J Neurosci. 2009 Jun 17;29(24):7886-97. doi: 10.1523/JNEUROSCI.0087-09.2009. J Neurosci. 2009. PMID: 19535600 Free PMC article.
-
Attractive axon guidance involves asymmetric membrane transport and exocytosis in the growth cone.Nat Neurosci. 2007 Jan;10(1):58-66. doi: 10.1038/nn1814. Epub 2006 Dec 10. Nat Neurosci. 2007. PMID: 17159991
-
Steering neuronal growth cones by shifting the imbalance between exocytosis and endocytosis.J Neurosci. 2014 May 21;34(21):7165-78. doi: 10.1523/JNEUROSCI.5261-13.2014. J Neurosci. 2014. PMID: 24849351 Free PMC article.
-
Microtubule dynamics in axon guidance.Neurosci Bull. 2014 Aug;30(4):569-83. doi: 10.1007/s12264-014-1444-6. Epub 2014 Jun 26. Neurosci Bull. 2014. PMID: 24968808 Free PMC article. Review.
-
Second messengers and membrane trafficking direct and organize growth cone steering.Nat Rev Neurosci. 2011 Apr;12(4):191-203. doi: 10.1038/nrn2996. Epub 2011 Mar 9. Nat Rev Neurosci. 2011. PMID: 21386859 Free PMC article. Review.
Cited by
-
An EPAC1/PDE1C-Signaling Axis Regulates Formation of Leading-Edge Protrusion in Polarized Human Arterial Vascular Smooth Muscle Cells.Cells. 2019 Nov 20;8(12):1473. doi: 10.3390/cells8121473. Cells. 2019. PMID: 31757003 Free PMC article.
-
FIGNL1 associates with KIF1Bβ and BICD1 to restrict dynein transport velocity during axon navigation.J Cell Biol. 2019 Oct 7;218(10):3290-3306. doi: 10.1083/jcb.201805128. Epub 2019 Sep 19. J Cell Biol. 2019. PMID: 31541015 Free PMC article.
-
Ogt Deficiency Induces Abnormal Cerebellar Function and Behavioral Deficits of Adult Mice through Modulating RhoA/ROCK Signaling.J Neurosci. 2023 Jun 21;43(25):4559-4579. doi: 10.1523/JNEUROSCI.1962-22.2023. Epub 2023 May 24. J Neurosci. 2023. PMID: 37225434 Free PMC article.
-
Molecular basis of the functions of the mammalian neuronal growth cone revealed using new methods.Proc Jpn Acad Ser B Phys Biol Sci. 2019;95(7):358-377. doi: 10.2183/pjab.95.026. Proc Jpn Acad Ser B Phys Biol Sci. 2019. PMID: 31406059 Free PMC article. Review.
-
In Vivo Live Imaging of Axonal Transport in Developing Zebrafish Axons.Methods Mol Biol. 2022;2431:325-350. doi: 10.1007/978-1-0716-1990-2_17. Methods Mol Biol. 2022. PMID: 35412285
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous
