Dysregulation of hepatic cAMP levels via altered Pde4b expression plays a critical role in alcohol-induced steatosis
- PMID: 27287961
- PMCID: PMC4993672
- DOI: 10.1002/path.4760
Dysregulation of hepatic cAMP levels via altered Pde4b expression plays a critical role in alcohol-induced steatosis
Abstract
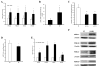
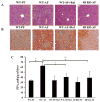
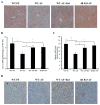
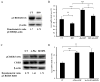
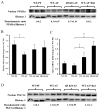
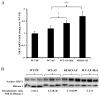
Alcohol-induced hepatic steatosis is a significant risk factor for progressive liver disease. Cyclic adenosine monophosphate (cAMP) signalling has been shown to significantly regulate lipid metabolism; however, the role of altered cAMP homeostasis in alcohol-mediated hepatic steatosis has never been studied. Our previous work demonstrated that increased expression of hepatic phosphodiesterase 4 (Pde4), which specifically hydrolyses and decreases cAMP levels, plays a pathogenic role in the development of liver inflammation/injury. The aim of this study was to examine the role of PDE4 in alcohol-induced hepatic steatosis. C57BL/6 wild-type and Pde4b knockout (Pde4b(-/-) ) mice were pair-fed control or ethanol liquid diets. One group of wild-type mice received rolipram, a PDE4-specific inhibitor, during alcohol feeding. We demonstrate for the first time that an early increase in PDE4 enzyme expression and a resultant decrease in hepatic cAMP levels are associated with the significant reduction in carnitine palmitoyltransferase 1A (Cpt1a) expression. Notably, alcohol-fed (AF) Pde4b(-/-) mice and AF wild-type mice treated with rolipram had significantly lower hepatic free fatty acid content compared with AF wild-type mice. Importantly, PDE4 inhibition in alcohol-fed mice prevented the decrease in hepatic Cpt1a expression via the Pparα/Sirt1/Pgc1α pathway. These results demonstrate that the alcohol- induced increase in hepatic Pde4, specifically Pde4b expression, and compromised cAMP signalling predispose the liver to impaired fatty acid oxidation and the development of steatosis. Moreover, these data also suggest that hepatic PDE4 may be a clinically relevant therapeutic _target for the treatment of alcohol-induced hepatic steatosis. Copyright © 2016 Pathological Society of Great Britain and Ireland. Published by John Wiley & Sons, Ltd.
Keywords: PDE4; PGC1α, SIRT1, CPT1A; PPARα; alcohol; cAMP; hepatic steatosis.
Copyright © 2016 Pathological Society of Great Britain and Ireland. Published by John Wiley & Sons, Ltd.
Conflict of interest statement
Authors declare no conflicts of interest
Figures
Similar articles
-
Phosphodiesterase 4 Inhibition as a Therapeutic _target for Alcoholic Liver Disease: From Bedside to Bench.Hepatology. 2019 Dec;70(6):1958-1971. doi: 10.1002/hep.30761. Epub 2019 Jun 25. Hepatology. 2019. PMID: 31081957 Free PMC article.
-
Phosphodiesterase 4b expression plays a major role in alcohol-induced neuro-inflammation.Neuropharmacology. 2017 Oct;125:376-385. doi: 10.1016/j.neuropharm.2017.08.011. Epub 2017 Aug 12. Neuropharmacology. 2017. PMID: 28807677 Free PMC article.
-
Phosphodiesterase 4B negatively regulates endotoxin-activated interleukin-1 receptor antagonist responses in macrophages.Sci Rep. 2017 Apr 6;7:46165. doi: 10.1038/srep46165. Sci Rep. 2017. PMID: 28383060 Free PMC article.
-
PET measurements of cAMP-mediated phosphodiesterase-4 with (R)-[11C]rolipram.Curr Radiopharm. 2011 Jan;4(1):44-58. doi: 10.2174/1874471011104010044. Curr Radiopharm. 2011. PMID: 22191614 Review.
-
Phosphodiesterase-4 inhibition as a therapeutic strategy for metabolic disorders.Obes Rev. 2016 May;17(5):429-41. doi: 10.1111/obr.12385. Epub 2016 Mar 21. Obes Rev. 2016. PMID: 26997580 Review.
Cited by
-
The Complexity and Multiplicity of the Specific cAMP Phosphodiesterase Family: PDE4, Open New Adapted Therapeutic Approaches.Int J Mol Sci. 2022 Sep 13;23(18):10616. doi: 10.3390/ijms231810616. Int J Mol Sci. 2022. PMID: 36142518 Free PMC article. Review.
-
Therapeutic _targeting of 3',5'-cyclic nucleotide phosphodiesterases: inhibition and beyond.Nat Rev Drug Discov. 2019 Oct;18(10):770-796. doi: 10.1038/s41573-019-0033-4. Epub 2019 Aug 6. Nat Rev Drug Discov. 2019. PMID: 31388135 Free PMC article. Review.
-
Phosphodiesterase 4 Inhibition as a Therapeutic _target for Alcoholic Liver Disease: From Bedside to Bench.Hepatology. 2019 Dec;70(6):1958-1971. doi: 10.1002/hep.30761. Epub 2019 Jun 25. Hepatology. 2019. PMID: 31081957 Free PMC article.
-
Role of cAMP and phosphodiesterase signaling in liver health and disease.Cell Signal. 2018 Sep;49:105-115. doi: 10.1016/j.cellsig.2018.06.005. Epub 2018 Jun 11. Cell Signal. 2018. PMID: 29902522 Free PMC article. Review.
-
Noninvasive 40-Hz Light Flicker Rescues Circadian Behavior and Abnormal Lipid Metabolism Induced by Acute Ethanol Exposure via Improving SIRT1 and the Circadian Clock in the Liver-Brain Axis.Front Pharmacol. 2020 Mar 25;11:355. doi: 10.3389/fphar.2020.00355. eCollection 2020. Front Pharmacol. 2020. PMID: 32269528 Free PMC article.
References
-
- An L, Wang X, Cederbaum AI. Cytokines in alcoholic liver disease. Arch Toxicol. 2012;86:1337–1348. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

