Pro-haloacetate Nanoparticles for Efficient Cancer Therapy via Pyruvate Dehydrogenase Kinase Modulation
- PMID: 27323896
- PMCID: PMC4914936
- DOI: 10.1038/srep28196
Pro-haloacetate Nanoparticles for Efficient Cancer Therapy via Pyruvate Dehydrogenase Kinase Modulation
Abstract
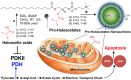
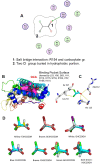
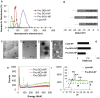
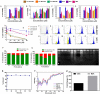
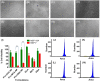
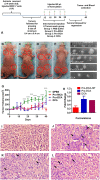
Anticancer agents based on haloacetic acids are developed for inhibition of pyruvate dehydrogenase kinase (PDK), an enzyme responsible for reversing the suppression of mitochondria-dependent apoptosis. Through molecular docking studies mono- and dihaloacetates are identified as potent PDK2 binders and matched their efficiency with dichloroacetic acid. In silico screening directed their conversion to phospholipid prodrugs, which were subsequently self-assembled to pro-haloacetate nanoparticles. Following a thorough physico-chemical characterization, the functional activity of these novel agents was established in wide ranges of human cancer cell lines in vitro and in vivo in rodents. Results indicated that the newly explored PDK modulators can act as efficient agent for cancer regression. A Pyruvate dehydrogenase (PDH) assay mechanistically confirmed that these agents trigger their activity through the mitochondria-dependent apoptosis.
Figures

Similar articles
-
Activation of mitochondrial oxidation by PDK2 inhibition reverses cisplatin resistance in head and neck cancer.Cancer Lett. 2016 Feb 1;371(1):20-9. doi: 10.1016/j.canlet.2015.11.023. Epub 2015 Nov 23. Cancer Lett. 2016. PMID: 26607904
-
Antitumor and chemosensitizing action of dichloroacetate implicates modulation of tumor microenvironment: a role of reorganized glucose metabolism, cell survival regulation and macrophage differentiation.Toxicol Appl Pharmacol. 2013 Nov 15;273(1):196-208. doi: 10.1016/j.taap.2013.09.005. Epub 2013 Sep 17. Toxicol Appl Pharmacol. 2013. PMID: 24051182
-
Discovery of the 3-Amino-1,2,4-triazine-Based Library as Selective PDK1 Inhibitors with Therapeutic Potential in Highly Aggressive Pancreatic Ductal Adenocarcinoma.Int J Mol Sci. 2023 Feb 12;24(4):3679. doi: 10.3390/ijms24043679. Int J Mol Sci. 2023. PMID: 36835086 Free PMC article.
-
Multi-modality imaging to assess metabolic response to dichloroacetate treatment in tumor models.Onco_target. 2016 Dec 6;7(49):81741-81749. doi: 10.18632/onco_target.13176. Onco_target. 2016. PMID: 28082726 Free PMC article.
-
Two dichloric compounds inhibit in vivo U87 xenograft tumor growth.Cancer Biol Ther. 2019;20(9):1281-1289. doi: 10.1080/15384047.2019.1632131. Epub 2019 Jun 24. Cancer Biol Ther. 2019. PMID: 31234707 Free PMC article.
Cited by
-
Use of acidic nanoparticles to rescue macrophage lysosomal dysfunction in atherosclerosis.Autophagy. 2023 Mar;19(3):886-903. doi: 10.1080/15548627.2022.2108252. Epub 2022 Aug 18. Autophagy. 2023. PMID: 35982578 Free PMC article.
-
Chimeric Drug Design with a Noncharged Carrier for Mitochondrial Delivery.Pharmaceutics. 2021 Feb 12;13(2):254. doi: 10.3390/pharmaceutics13020254. Pharmaceutics. 2021. PMID: 33673228 Free PMC article.
-
Pro-Nifuroxazide Self-Assembly Leads to Triggerable Nanomedicine for Anti-cancer Therapy.ACS Appl Mater Interfaces. 2019 May 22;11(20):18074-18089. doi: 10.1021/acsami.9b01343. Epub 2019 May 13. ACS Appl Mater Interfaces. 2019. PMID: 31013055 Free PMC article.
-
The reversed intra- and extracellular pH in tumors as a unified strategy to chemotherapeutic delivery using _targeted nanocarriers.Acta Pharm Sin B. 2021 Aug;11(8):2243-2264. doi: 10.1016/j.apsb.2021.01.012. Epub 2021 Jan 24. Acta Pharm Sin B. 2021. PMID: 34522586 Free PMC article. Review.
-
Caffeic Acid Expands Anti-Tumor Effect of Metformin in Human Metastatic Cervical Carcinoma HTB-34 Cells: Implications of AMPK Activation and Impairment of Fatty Acids De Novo Biosynthesis.Int J Mol Sci. 2017 Feb 21;18(2):462. doi: 10.3390/ijms18020462. Int J Mol Sci. 2017. PMID: 28230778 Free PMC article.
References
-
- Bonnet S. et al.. A mitochondria-K+ channel axis is suppressed in cancer and its normalization promotes apoptosis and inhibits cancer growth. Cancer Cell 11, 37–51 (2007). - PubMed
-
- Welsh S., Williams R., Kirkpatrick L. & Powis G. Antitumor activity and pharmacodynamic properties of PX-478, an inhibitor of hypoxia-inducible factor-1alpha. Mol. Cancer Ther. 3, 233–244 (2004). - PubMed
-
- Deus C. M., Coelho A. R., Serafim T. L. & Oliveira P. J. _targeting mitochondrial function for the treatment of breast cancer. Fut. Med. Chem. 6, 1499–1513 (2014). - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

