Chronic Cognitive Dysfunction after Traumatic Brain Injury Is Improved with a Phosphodiesterase 4B Inhibitor
- PMID: 27383587
- PMCID: PMC4938858
- DOI: 10.1523/JNEUROSCI.3212-15.2016
Chronic Cognitive Dysfunction after Traumatic Brain Injury Is Improved with a Phosphodiesterase 4B Inhibitor
Abstract
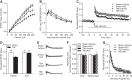
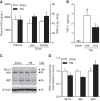
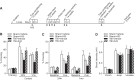
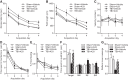
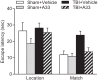
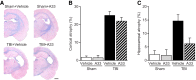
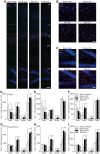
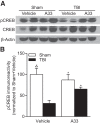
Learning and memory impairments are common in traumatic brain injury (TBI) survivors. However, there are no effective treatments to improve TBI-induced learning and memory impairments. TBI results in decreased cAMP signaling and reduced cAMP-response-element binding protein (CREB) activation, a critical pathway involved in learning and memory. TBI also acutely upregulates phosphodiesterase 4B2 (PDE4B2), which terminates cAMP signaling by hydrolyzing cAMP. We hypothesized that a subtype-selective PDE4B inhibitor could reverse the learning deficits induced by TBI. To test this hypothesis, adult male Sprague-Dawley rats received sham surgery or moderate parasagittal fluid-percussion brain injury. At 3 months postsurgery, animals were administered a selective PDE4B inhibitor or vehicle before cue and contextual fear conditioning, water maze training and a spatial working memory task. Treatment with the PDE4B inhibitor significantly reversed the TBI-induced deficits in cue and contextual fear conditioning and water maze retention. To further understand the underlying mechanisms of these memory impairments, we examined hippocampal long-term potentiation (LTP). TBI resulted in a significant reduction in basal synaptic transmission and impaired expression of LTP. Treatment with the PDE4B inhibitor significantly reduced the deficits in basal synaptic transmission and rescued LTP expression. The PDE4B inhibitor reduced tumor necrosis factor-α levels and increased phosphorylated CREB levels after TBI, suggesting that this drug inhibited molecular pathways in the brain known to be regulated by PDE4B. These results suggest that a subtype-selective PDE4B inhibitor is a potential therapeutic to reverse chronic learning and memory dysfunction and deficits in hippocampal synaptic plasticity following TBI.
Significance statement: Currently, there are an estimated 3.2-5.3 million individuals living with disabilities from traumatic brain injury (TBI) in the United States, and 8 of 10 of these individuals report cognitive disabilities (Thurman et al., 1999; Lew et al., 2006; Zaloshnja et al., 2008). One of the molecular mechanisms associated with chronic cognitive disabilities is impaired cAMP signaling in the hippocampus. In this study, we report that a selective phosphodiesterase 4B (PDE4B) inhibitor reduces chronic cognitive deficits after TBI and rescues deficits in hippocampal long-term potentiation. These results suggest that PDE4B inhibition has the potential to improve learning and memory ability and overall functioning for people living with TBI.
Keywords: cAMP; cognition; learning; long-term potentiation; phosphodiesterase; traumatic brain injury.
Copyright © 2016 the authors 0270-6474/16/367095-14$15.00/0.
Figures
Comment in
-
Tumor Necrosis Factor α as a Potential Mediator of the Effects of Phosphodiesterase 4B Inhibition on Cognition after Traumatic Brain Injury.J Neurosci. 2016 Nov 16;36(46):11587-11589. doi: 10.1523/JNEUROSCI.2799-16.2016. J Neurosci. 2016. PMID: 27852768 Free PMC article. No abstract available.
Similar articles
-
A negative allosteric modulator of PDE4D enhances learning after traumatic brain injury.Neurobiol Learn Mem. 2018 Feb;148:38-49. doi: 10.1016/j.nlm.2017.12.008. Epub 2017 Dec 30. Neurobiol Learn Mem. 2018. PMID: 29294383 Free PMC article.
-
Phosphodiesterase inhibition rescues chronic cognitive deficits induced by traumatic brain injury.J Neurosci. 2013 Mar 20;33(12):5216-26. doi: 10.1523/JNEUROSCI.5133-12.2013. J Neurosci. 2013. PMID: 23516287 Free PMC article.
-
Positive allosteric modulation of the α7 nicotinic acetylcholine receptor as a treatment for cognitive deficits after traumatic brain injury.PLoS One. 2019 Oct 3;14(10):e0223180. doi: 10.1371/journal.pone.0223180. eCollection 2019. PLoS One. 2019. PMID: 31581202 Free PMC article.
-
Therapeutic benefits of phosphodiesterase 4B inhibition after traumatic brain injury.PLoS One. 2017 May 19;12(5):e0178013. doi: 10.1371/journal.pone.0178013. eCollection 2017. PLoS One. 2017. PMID: 28542295 Free PMC article.
-
Behavioral Assays for Comprehensive Evaluation of Cognitive and Neuropsychiatric Comorbidities of Traumatic Brain Injury and Chronic Neurological Disorders.Curr Protoc. 2024 Oct;4(10):e70019. doi: 10.1002/cpz1.70019. Curr Protoc. 2024. PMID: 39422165 Review.
Cited by
-
Selective Inhibition of PDE4B Reduces Binge Drinking in Two C57BL/6 Substrains.Int J Mol Sci. 2021 May 21;22(11):5443. doi: 10.3390/ijms22115443. Int J Mol Sci. 2021. PMID: 34064099 Free PMC article.
-
Dominant-Negative Attenuation of cAMP-Selective Phosphodiesterase PDE4D Action Affects Learning and Behavior.Int J Mol Sci. 2020 Aug 9;21(16):5704. doi: 10.3390/ijms21165704. Int J Mol Sci. 2020. PMID: 32784895 Free PMC article.
-
A Mouse Model of Hepatic Ischemia-Reperfusion Injury Demonstrates Potentially Reversible Effects on Hippocampal Neurons and Postoperative Cognitive Function.Med Sci Monit. 2019 Feb 27;25:1526-1536. doi: 10.12659/MSM.912658. Med Sci Monit. 2019. PMID: 30808858 Free PMC article.
-
Transcriptomic and bioinformatics analysis of the mechanism by which erythropoietin promotes recovery from traumatic brain injury in mice.Neural Regen Res. 2024 Jan;19(1):171-179. doi: 10.4103/1673-5374.374135. Neural Regen Res. 2024. PMID: 37488864 Free PMC article.
-
A Nonhuman Primate PET Study: Measurement of Brain PDE4 Occupancy by Roflumilast Using (R)-[11C]Rolipram.Mol Imaging Biol. 2018 Aug;20(4):615-622. doi: 10.1007/s11307-018-1168-0. Mol Imaging Biol. 2018. PMID: 29441434
References
-
- Acosta SA, Tajiri N, Shinozuka K, Ishikawa H, Grimmig B, Diamond D, Sanberg PR, Bickford PC, Kaneko Y, Borlongan CV. Long-term upregulation of inflammation and suppression of cell proliferation in the brain of adult rats exposed to traumatic brain injury using the controlled cortical impact model. PLoS One. 2013;8:e53376. doi: 10.1371/journal.pone.0053376. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
