Sex hormonal regulation of cardiac ion channels in drug-induced QT syndromes
- PMID: 27595633
- PMCID: PMC5140718
- DOI: 10.1016/j.pharmthera.2016.09.004
Sex hormonal regulation of cardiac ion channels in drug-induced QT syndromes
Abstract
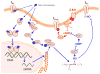
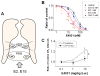
Female sex is an independent risk factor for development of torsade de pointes (TdP) arrhythmias not only in congenital long QT syndromes but also in acquired long QT syndromes. Clinical and experimental evidences suggest that the gender differences may be due to, at least in part, gender differences in regulation of rate-corrected QT (QTC) interval between men and women. In adult women, both QTC interval and arrhythmic risks in TdP alter cyclically during menstrual cycle, suggesting a critical role of female sex hormones in cardiac repolarization process. These gender differences in fundamental cardiac electrophysiology result from variable ion channel expression and diverse sex hormonal regulation via long term genomic and acute non-genomic actions, and sex differences in drug responses and metabolisms. In particular, non-genomic actions of testosterone and progesterone on cardiac ion channels are likely to contribute to the gender differences in cardiac repolarization processes. This review summarizes current knowledge on sex hormonal regulation of cardiac ion channels which contribute to cardiac repolarization processes and its implication for gender differences in drug-induced long QT syndromes.
Keywords: Arrhythmias; Cardiac repolarization; Gender difference; Sex hormones.
Copyright © 2016 Elsevier Inc. All rights reserved.
Conflict of interest statement
Statement The authors declare that there are no conflicts of interest.
Figures
Similar articles
-
Non-genomic action of sex steroid hormones and cardiac repolarization.Biol Pharm Bull. 2013;36(1):8-12. doi: 10.1248/bpb.b212021. Biol Pharm Bull. 2013. PMID: 23302631 Review.
-
Influence of steroid hormones on ventricular repolarization.Pharmacol Ther. 2016 Nov;167:38-47. doi: 10.1016/j.pharmthera.2016.07.005. Epub 2016 Jul 22. Pharmacol Ther. 2016. PMID: 27452340 Review.
-
Sex differences in ventricular repolarization: from cardiac electrophysiology to Torsades de Pointes.Fundam Clin Pharmacol. 2004 Apr;18(2):139-51. doi: 10.1111/j.1472-8206.2004.00230.x. Fundam Clin Pharmacol. 2004. PMID: 15066127 Review.
-
Regulation of cardiac ion channels via non-genomic action of sex steroid hormones: implication for the gender difference in cardiac arrhythmias.Pharmacol Ther. 2007 Jul;115(1):106-15. doi: 10.1016/j.pharmthera.2007.04.008. Epub 2007 May 13. Pharmacol Ther. 2007. PMID: 17583354 Review.
-
Drugs for men and women - how important is gender as a risk factor for TdP?Pharmacol Ther. 2008 Aug;119(2):186-94. doi: 10.1016/j.pharmthera.2008.03.005. Epub 2008 Apr 7. Pharmacol Ther. 2008. PMID: 18472167
Cited by
-
Sudden Death in Men Versus Women with Heart Failure.Curr Heart Fail Rep. 2023 Apr;20(2):129-137. doi: 10.1007/s11897-023-00596-z. Epub 2023 Mar 7. Curr Heart Fail Rep. 2023. PMID: 36881322 Review.
-
Stratification by Sex and Hormone Level When Contrasting Men and Women in Schizophrenia Trials Will Improve Personalized Treatment.J Pers Med. 2021 Sep 18;11(9):929. doi: 10.3390/jpm11090929. J Pers Med. 2021. PMID: 34575706 Free PMC article. Review.
-
Pediatric sex estimation using AI-enabled ECG analysis: influence of pubertal development.NPJ Digit Med. 2024 Jul 2;7(1):176. doi: 10.1038/s41746-024-01165-x. NPJ Digit Med. 2024. PMID: 38956410 Free PMC article.
-
Investigation of the Effects of Cardiovascular Therapeutic Ultrasound Applied in Female and Male Rats' Hearts of Different Ages.IEEE Trans Ultrason Ferroelectr Freq Control. 2022 Jan;69(1):166-180. doi: 10.1109/TUFFC.2021.3113867. Epub 2021 Dec 31. IEEE Trans Ultrason Ferroelectr Freq Control. 2022. PMID: 34543195 Free PMC article.
-
Age and Sex Estimation Using Artificial Intelligence From Standard 12-Lead ECGs.Circ Arrhythm Electrophysiol. 2019 Sep;12(9):e007284. doi: 10.1161/CIRCEP.119.007284. Epub 2019 Aug 27. Circ Arrhythm Electrophysiol. 2019. PMID: 31450977 Free PMC article.
References
-
- Abi-Gerges N, Philp K, Pollard C, Wakefield I, Hammond TG, Valentin JP. Sex differences in ventricular repolarization: from cardiac electrophysiology to Torsades de Pointes. Fundam Clin Pharmacol. 2004;18(2):139–151. - PubMed
-
- Abriel H, Schlapfer J, Keller DI, Gavillet B, Buclin T, Biollaz J, et al. Molecular and clinical determinants of drug-induced long QT syndrome: an iatrogenic channelopathy. Swiss Med Wkly. 2004;134(47–48):685–694. - PubMed
-
- Asada K, Kurokawa J, Furukawa T. Redox- and Calmodulin-dependent S-Nitrosylation of the KCNQ1 Channel. The Journal of biological chemistry. 2009;284(9):6014–6020. - PubMed
-
- Bai CX, Kurokawa J, Tamagawa M, Nakaya H, Furukawa T. Nontranscriptional regulation of cardiac repolarization currents by testosterone. Circulation. 2005;112(12):1701–1710. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

