Back to the tubule: microtubule dynamics in Parkinson's disease
- PMID: 27600680
- PMCID: PMC5241350
- DOI: 10.1007/s00018-016-2351-6
Back to the tubule: microtubule dynamics in Parkinson's disease
Abstract
Cytoskeletal homeostasis is essential for the development, survival and maintenance of an efficient nervous system. Microtubules are highly dynamic polymers important for neuronal growth, morphology, migration and polarity. In cooperation with several classes of binding proteins, microtubules regulate long-distance intracellular cargo trafficking along axons and dendrites. The importance of a delicate interplay between cytoskeletal components is reflected in several human neurodegenerative disorders linked to abnormal microtubule dynamics, including Parkinson's disease (PD). Mounting evidence now suggests PD pathogenesis might be underlined by early cytoskeletal dysfunction. Advances in genetics have identified PD-associated mutations and variants in genes encoding various proteins affecting microtubule function including the microtubule-associated protein tau. In this review, we highlight the role of microtubules, their major posttranslational modifications and microtubule associated proteins in neuronal function. We then present key evidence on the contribution of microtubule dysfunction to PD. Finally, we discuss how regulation of microtubule dynamics with microtubule-_targeting agents and deacetylase inhibitors represents a promising strategy for innovative therapeutic development.
Keywords: Axonal transport; Cytoskeleton; LRRK2; Microtubule dynamics; Microtubule _targeting agents; PARK genes; Parkinson’s disease; Tau; Wnt signalling.
Figures

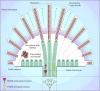



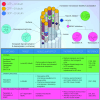
Similar articles
-
Microtubule Dysfunction: A Common Feature of Neurodegenerative Diseases.Int J Mol Sci. 2020 Oct 5;21(19):7354. doi: 10.3390/ijms21197354. Int J Mol Sci. 2020. PMID: 33027950 Free PMC article. Review.
-
Regulation of neuronal microtubule dynamics by tau: Implications for tauopathies.Int J Biol Macromol. 2019 Jul 15;133:473-483. doi: 10.1016/j.ijbiomac.2019.04.120. Epub 2019 Apr 17. Int J Biol Macromol. 2019. PMID: 31004638 Review.
-
Acetylation as a major determinant to microtubule-dependent autophagy: Relevance to Alzheimer's and Parkinson disease pathology.Biochim Biophys Acta Mol Basis Dis. 2019 Aug 1;1865(8):2008-2023. doi: 10.1016/j.bbadis.2018.11.014. Epub 2018 Dec 17. Biochim Biophys Acta Mol Basis Dis. 2019. PMID: 30572013
-
Neuronal microtubules and proteins linked to Parkinson's disease: a relevant interaction?Biol Chem. 2019 Aug 27;400(9):1099-1112. doi: 10.1515/hsz-2019-0142. Biol Chem. 2019. PMID: 31256059 Review.
-
Tubulin post-translational modifications: encoding functions on the neuronal microtubule cytoskeleton.Trends Neurosci. 2010 Aug;33(8):362-72. doi: 10.1016/j.tins.2010.05.001. Epub 2010 Jun 11. Trends Neurosci. 2010. PMID: 20541813 Review.
Cited by
-
Computational Approaches to the Rational Design of Tubulin-_targeting Agents.Biomolecules. 2023 Feb 2;13(2):285. doi: 10.3390/biom13020285. Biomolecules. 2023. PMID: 36830654 Free PMC article. Review.
-
In vitro Characterization of Gut Microbiota-Derived Bacterial Strains With Neuroprotective Properties.Front Cell Neurosci. 2019 Sep 20;13:402. doi: 10.3389/fncel.2019.00402. eCollection 2019. Front Cell Neurosci. 2019. PMID: 31619962 Free PMC article.
-
Linking acetylated α-Tubulin redistribution to α-Synuclein pathology in brain of Parkinson's disease patients.NPJ Parkinsons Dis. 2024 Jan 2;10(1):2. doi: 10.1038/s41531-023-00607-9. NPJ Parkinsons Dis. 2024. PMID: 38167511 Free PMC article.
-
Aggregation of alpha-synuclein in enteric neurons does not impact function in vitro.Sci Rep. 2022 Dec 23;12(1):22211. doi: 10.1038/s41598-022-26543-x. Sci Rep. 2022. PMID: 36564445 Free PMC article.
-
Alterations in Cerebellar Microtubule Cytoskeletal Network in a ValproicAcid-Induced Rat Model of Autism Spectrum Disorders.Biomedicines. 2022 Nov 24;10(12):3031. doi: 10.3390/biomedicines10123031. Biomedicines. 2022. PMID: 36551785 Free PMC article.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

