Suppressor of Cytokine Signaling-1 Peptidomimetic Limits Progression of Diabetic Nephropathy
- PMID: 27609616
- PMCID: PMC5280022
- DOI: 10.1681/ASN.2016020237
Suppressor of Cytokine Signaling-1 Peptidomimetic Limits Progression of Diabetic Nephropathy
Abstract
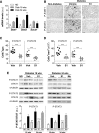
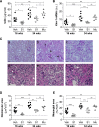
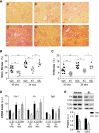
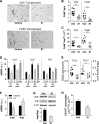
Diabetes is the main cause of CKD and ESRD worldwide. Chronic activation of Janus kinase and signal transducer and activator of transcription (STAT) signaling contributes to diabetic nephropathy by inducing genes involved in leukocyte infiltration, cell proliferation, and extracellular matrix accumulation. This study examined whether a cell-permeable peptide mimicking the kinase-inhibitory region of suppressor of cytokine signaling-1 (SOCS1) regulatory protein protects against nephropathy by suppressing STAT-mediated cell responses to diabetic conditions. In a mouse model combining hyperglycemia and hypercholesterolemia (streptozotocin diabetic, apoE-deficient mice), renal STAT activation status correlated with the severity of nephropathy. Notably, compared with administration of vehicle or mutant inactive peptide, administration of the SOCS1 peptidomimetic at either early or advanced stages of diabetes ameliorated STAT activity and resulted in reduced serum creatinine level, albuminuria, and renal histologic changes (mesangial expansion, tubular injury, and fibrosis) over time. Mice treated with the SOCS1 peptidomimetic also exhibited reduced kidney leukocyte recruitment (T lymphocytes and classic M1 proinflammatory macrophages) and decreased expression levels of proinflammatory and profibrotic markers that were independent of glycemic and lipid changes. In vitro, internalized peptide suppressed STAT activation and _target gene expression induced by inflammatory and hyperglycemic conditions, reduced migration and proliferation in mesangial and tubuloepithelial cells, and altered the expression of cytokine-induced macrophage polarization markers. In conclusion, our study identifies SOCS1 mimicking as a feasible therapeutic strategy to halt the onset and progression of renal inflammation and fibrosis in diabetic kidney disease.
Keywords: Chronic inflammation; apolipoprotein E; diabetic nephropathy; fibrosis; macrophages; transcription factors.
Copyright © 2017 by the American Society of Nephrology.
Figures

Similar articles
-
SOCS1-_targeted therapy ameliorates renal and vascular oxidative stress in diabetes via STAT1 and PI3K inhibition.Lab Invest. 2018 Oct;98(10):1276-1290. doi: 10.1038/s41374-018-0043-6. Epub 2018 Mar 14. Lab Invest. 2018. PMID: 29540859
-
Suppressor of cytokine signaling 1-derived peptide inhibits Janus kinase/signal transducers and activators of transcription pathway and improves inflammation and atherosclerosis in diabetic mice.Arterioscler Thromb Vasc Biol. 2014 Sep;34(9):1953-60. doi: 10.1161/ATVBAHA.114.304144. Epub 2014 Jul 10. Arterioscler Thromb Vasc Biol. 2014. PMID: 25012131
-
SOCS1 Peptidomimetic Alleviates Glomerular Inflammation in MsPGN by Inhibiting Macrophage M1 Polarization.Inflammation. 2023 Dec;46(6):2402-2414. doi: 10.1007/s10753-023-01886-3. Epub 2023 Aug 15. Inflammation. 2023. PMID: 37581761
-
_targeting Macrophages: Therapeutic Approaches in Diabetic Kidney Disease.Int J Mol Sci. 2024 Apr 15;25(8):4350. doi: 10.3390/ijms25084350. Int J Mol Sci. 2024. PMID: 38673935 Free PMC article. Review.
-
Novel Anti-inflammatory and Anti-fibrotic Agents for Diabetic Kidney Disease-From Bench to Bedside.Adv Chronic Kidney Dis. 2021 Jul;28(4):378-390. doi: 10.1053/j.ackd.2021.09.010. Adv Chronic Kidney Dis. 2021. PMID: 34922694 Review.
Cited by
-
A mutual regulatory loop between miR-155 and SOCS1 influences renal inflammation and diabetic kidney disease.Mol Ther Nucleic Acids. 2023 Sep 27;34:102041. doi: 10.1016/j.omtn.2023.102041. eCollection 2023 Dec 12. Mol Ther Nucleic Acids. 2023. PMID: 37842165 Free PMC article.
-
Sitagliptin Mitigates Diabetic Nephropathy in a Rat Model of Streptozotocin-Induced Type 2 Diabetes: Possible Role of PTP1B/JAK-STAT Pathway.Int J Mol Sci. 2023 Mar 31;24(7):6532. doi: 10.3390/ijms24076532. Int J Mol Sci. 2023. PMID: 37047505 Free PMC article.
-
Structure-Activity Relationship Investigations of Novel Constrained Chimeric Peptidomimetics of SOCS3 Protein _targeting JAK2.Pharmaceuticals (Basel). 2022 Apr 9;15(4):458. doi: 10.3390/ph15040458. Pharmaceuticals (Basel). 2022. PMID: 35455455 Free PMC article.
-
Proteomimetics of Natural Regulators of JAK-STAT Pathway: Novel Therapeutic Perspectives.Front Mol Biosci. 2022 Jan 3;8:792546. doi: 10.3389/fmolb.2021.792546. eCollection 2021. Front Mol Biosci. 2022. PMID: 35047557 Free PMC article. Review.
-
Activation of the NLRP3 inflammasome by RAC1 mediates a new mechanism in diabetic nephropathy.Inflamm Res. 2022 Feb;71(2):191-204. doi: 10.1007/s00011-021-01532-4. Epub 2022 Jan 14. Inflamm Res. 2022. PMID: 35028708
References
-
- Fineberg D, Jandeleit-Dahm KA, Cooper ME: Diabetic nephropathy: diagnosis and treatment. Nat Rev Endocrinol 9: 713–723, 2013 - PubMed
-
- Wada J, Makino H: Inflammation and the pathogenesis of diabetic nephropathy. Clin Sci (Lond) 124: 139–152, 2013 - PubMed
-
- Navarro-González JF, Mora-Fernández C, Muros de Fuentes M, García-Pérez J: Inflammatory molecules and pathways in the pathogenesis of diabetic nephropathy. Nat Rev Nephrol 7: 327–340, 2011 - PubMed
-
- Tuttle KR, Bakris GL, Bilous RW, Chiang JL, de Boer IH, Goldstein-Fuchs J, Hirsch IB, Kalantar-Zadeh K, Narva AS, Navaneethan SD, Neumiller JJ, Patel UD, Ratner RE, Whaley-Connell AT, Molitch ME: Diabetic kidney disease: a report from an ADA Consensus Conference. Diabetes Care 37: 2864–2883, 2014 - PMC - PubMed
-
- Fernandez-Fernandez B, Ortiz A, Gomez-Guerrero C, Egido J: Therapeutic approaches to diabetic nephropathy--beyond the RAS. Nat Rev Nephrol 10: 325–346, 2014 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

