Ionic immune suppression within the tumour microenvironment limits T cell effector function
- PMID: 27626381
- PMCID: PMC5204372
- DOI: 10.1038/nature19364
Ionic immune suppression within the tumour microenvironment limits T cell effector function
Abstract
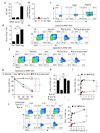
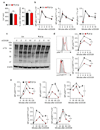
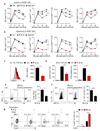
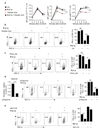
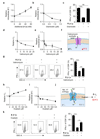
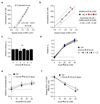
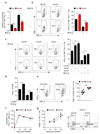
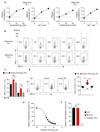
Tumours progress despite being infiltrated by tumour-specific effector T cells. Tumours contain areas of cellular necrosis, which are associated with poor survival in a variety of cancers. Here, we show that necrosis releases intracellular potassium ions into the extracellular fluid of mouse and human tumours, causing profound suppression of T cell effector function. Elevation of the extracellular potassium concentration ([K+]e) impairs T cell receptor (TCR)-driven Akt-mTOR phosphorylation and effector programmes. Potassium-mediated suppression of Akt-mTOR signalling and T cell function is dependent upon the activity of the serine/threonine phosphatase PP2A. Although the suppressive effect mediated by elevated [K+]e is independent of changes in plasma membrane potential (Vm), it requires an increase in intracellular potassium ([K+]i). Accordingly, augmenting potassium efflux in tumour-specific T cells by overexpressing the potassium channel Kv1.3 lowers [K+]i and improves effector functions in vitro and in vivo and enhances tumour clearance and survival in melanoma-bearing mice. These results uncover an ionic checkpoint that blocks T cell function in tumours and identify potential new strategies for cancer immunotherapy.
Conflict of interest statement
The authors declare no competing financial interests.
Figures

Comment in
-
Immunology: Channelling potassium to fight cancer.Nature. 2016 Sep 22;537(7621):497-499. doi: 10.1038/nature19467. Epub 2016 Sep 14. Nature. 2016. PMID: 27626384 No abstract available.
-
Potassium channels of T lymphocytes take center stage in the fight against cancer.J Immunother Cancer. 2017 Jan 17;5:2. doi: 10.1186/s40425-016-0202-5. eCollection 2017. J Immunother Cancer. 2017. PMID: 28105369 Free PMC article.
Similar articles
-
The cajanine derivative LJ101019C regulates the proliferation and enhances the activity of NK cells via Kv1.3 channel-driven activation of the AKT/mTOR pathway.Phytomedicine. 2020 Jan;66:153113. doi: 10.1016/j.phymed.2019.153113. Epub 2019 Nov 4. Phytomedicine. 2020. PMID: 31790901
-
PTEN loss correlates with T cell exclusion across human cancers.BMC Cancer. 2021 Apr 19;21(1):429. doi: 10.1186/s12885-021-08114-x. BMC Cancer. 2021. PMID: 33874915 Free PMC article.
-
PI3Kγ is a molecular switch that controls immune suppression.Nature. 2016 Nov 17;539(7629):437-442. doi: 10.1038/nature19834. Epub 2016 Sep 19. Nature. 2016. PMID: 27642729 Free PMC article.
-
Regulatory T cells in melanoma: the final hurdle towards effective immunotherapy?Lancet Oncol. 2012 Jan;13(1):e32-42. doi: 10.1016/S1470-2045(11)70155-3. Lancet Oncol. 2012. PMID: 22225723 Review.
-
Immune surveillance in melanoma: From immune attack to melanoma escape and even counterattack.Cancer Biol Ther. 2017 Jul 3;18(7):451-469. doi: 10.1080/15384047.2017.1323596. Epub 2017 May 17. Cancer Biol Ther. 2017. PMID: 28513269 Free PMC article. Review.
Cited by
-
The role of cuproptosis in gastric cancer.Front Immunol. 2024 Oct 30;15:1435651. doi: 10.3389/fimmu.2024.1435651. eCollection 2024. Front Immunol. 2024. PMID: 39539553 Free PMC article. Review.
-
Excess Potassium Promotes Autophagy to Maintain the Immunosuppressive Capacity of Myeloid-Derived Suppressor Cells Independent of Arginase 1.Cells. 2024 Oct 19;13(20):1736. doi: 10.3390/cells13201736. Cells. 2024. PMID: 39451254 Free PMC article.
-
Cutaneous adaptive immunity and uraemia: a narrative review.Front Immunol. 2024 Sep 27;15:1464338. doi: 10.3389/fimmu.2024.1464338. eCollection 2024. Front Immunol. 2024. PMID: 39399503 Free PMC article. Review.
-
Pancreatic cancer cells overexpressing interleukin 6 induce T-cell-mediated tumor clearance and durable anti-tumor immune response.bioRxiv [Preprint]. 2024 Sep 27:2024.09.26.615308. doi: 10.1101/2024.09.26.615308. bioRxiv. 2024. PMID: 39386578 Free PMC article. Preprint.
-
Identifying metabolic limitations in the tumor microenvironment.Sci Adv. 2024 Oct 4;10(40):eadq7305. doi: 10.1126/sciadv.adq7305. Epub 2024 Oct 2. Sci Adv. 2024. PMID: 39356752 Free PMC article. Review.
References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

